Total synthesis and evaluation of iso-duocarmycin SA and iso-yatakemycin
- PMID: 19154178
- PMCID: PMC2646182
- DOI: 10.1021/ja808108q
Total synthesis and evaluation of iso-duocarmycin SA and iso-yatakemycin
Erratum in
- J Am Chem Soc. 2014 Dec 3;136(48):16947. Nguyen, Trihn [corrected to Nguyen, Trinh]
Abstract
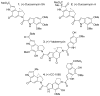
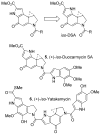
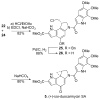
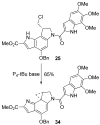
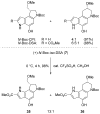
The total synthesis and evaluation of iso-duocarmycin SA (5) and iso-yatakemycin (6), representing key analogues of the corresponding natural products incorporating an isomeric alkylation subunit, are detailed. This pyrrole isomer of the natural alkylation subunit displayed an enhanced reaction regioselectivity and a 2-fold diminished stability. Although still exceptionally potent, the iso-duocarmycin SA derivatives and natural product analogues exhibited a corresponding approximate 3-5-fold reduction in cytotoxic activity [L1210 IC(50) for (+)-iso-duocarmycin SA = 50 pM and for (+)-iso-yatakemycin = 15 pM] consistent with their placement on a parabolic relationship correlating activity with reactivity. The DNA alkylation selectivity of the resulting key natural product analogues was unaltered by the structure modification in spite of the minor-groove presentation of a potential H-bond donor. Additionally, a unique ortho-spirocyclization with such derivatives was explored via the preparation, characterization, and evaluation of 34 that is incapable of the more conventional para-spirocyclization. Although 34 proved sufficiently stable for isolation and characterization, it displayed little stability in protic solvents (t(1/2) = 0.19 h at pH 3, t(1/2) = 0.20 h at pH 7), a pH-independent (H(+) independent) solvolysis rate profile at pH 3/4-7, and a much reduced cytotoxic potency, but a DNA alkylation selectivity and efficiency comparable to those of duocarmycin SA and iso-duocarmycin SA. The implications of these observations on the source of the DNA alkylation selectivity and catalysis for this class of natural products are discussed.
Figures






References
-
- Ichimura M, Ogawa T, Takahashi K, Kobayashi E, Kawamoto I, Yasuzawa T, Takahashi I, Nakano H. J Antibiot. 1990;43:1037–1038. - PubMed
-
-
Igarashi Y, Futamata K, Fujita T, Sekine A, Senda H, Naoki H, Furumai T. J Antibiot. 2003;56:107–113.Structure revision: Tichenor MS, Kastrinsky DB, Boger DL. J Am Chem Soc. 2004;126:8396–8398.
-
-
- Martin DG, Biles C, Gerpheide SA, Hanka LJ, Krueger WC, McGovren JP, Mizsak SA, Neil GL, Stewart JC, Visser J. J Antibiot. 1981;34:1119–1125. - PubMed
-
- Takahashi I, Takahashi K, Ichimura M, Morimoto M, Asano K, Kawamoto I, Tomita F, Nakano H. J Antibiot. 1988;41:1915–1917. - PubMed
-
- Duocarmycin SA, Boger DL, Johnson DS, Yun W. J Am Chem Soc. 1994;116:1635–1656.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

