The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300
- PMID: 19154186
- PMCID: PMC2657196
- DOI: 10.1111/j.1469-0691.2008.02648.x
The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300
Abstract
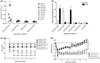
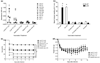
Methicillin-resistant Staphylococcus aureus is increasingly responsible for staphylococcal infections in the community. A large percentage of the community-acquired methicillin-resistant (CA-MRSA) strains in the USA produce Panton-Valentine leukocidin (PVL), which is associated with severe infections. The virulence of the clinical CA-MRSA strain USA300 was compared to that of its isogenic pvl-deleted mutant, and it was shown that PVL contributes to lung and muscle tissue destruction, respectively, in murine necrotizing pneumonia and skin infection models. Mice infected with the USA300 strain developed a dominant anti-PVL response. The PVL subunits were therefore tested as vaccinogens against this isolate, and their vaccine efficacy correlated with both the route of vaccination and infection. These data suggest that PVL is a virulence factor in murine CA-MRSA infections.
Figures



References
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. - PubMed
-
- Gonzalez BE, Hulten KG, Dishop MK, et al. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005;41:583–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

