Inhibition of heat shock protein 90 sensitizes melanoma cells to thermosensitive ferromagnetic particle-mediated hyperthermia with low Curie temperature
- PMID: 19154416
- PMCID: PMC11159285
- DOI: 10.1111/j.1349-7006.2008.01072.x
Inhibition of heat shock protein 90 sensitizes melanoma cells to thermosensitive ferromagnetic particle-mediated hyperthermia with low Curie temperature
Abstract
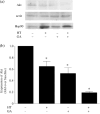
Heat shock protein (Hsp) 90 is a key regulator of a variety of oncogene products and cell-signaling molecules, and the therapeutic benefit of its inhibition in combination with radiation or chemotherapy has been investigated. In addition, hyperthermia has been used for many years to treat various malignant tumors. We previously described a system in which hyperthermia was induced using thermosensitive ferromagnetic particles (FMP) with a Curie temperature (Tc = 43 degrees C) low enough to mediate automatic temperature control, and demonstrated its antitumor effect in a mouse melanoma model. In the present study, we examined the antitumor effects of combining a Hsp90 inhibitor (geldanamycin; GA) with FMP-mediated hyperthermia. In cultured B16 melanoma cells, GA exerted an antitumor effect by increasing the cells' susceptibility to hyperthermia and reducing expression of Akt. In an in vivo study, melanoma cells were subcutaneously injected into the backs of C57BL/6 mice. FMP were then injected into the resultant tumors, and the mice were divided into four groups: group I, no treatment (control); group II, one hyperthermia treatment; group III, GA alone; and group IV, GA with hyperthermia. When exposed to a magnetic field, the temperature of tissues containing FMP increased and stabilized at the Tc. In group IV, complete regression of tumors was observed in five of nine mice (56%), whereas no tumor regression was seen in groups I-III. Our findings suggest that inhibition of Hsp90 with hyperthermia increases its antitumor effect. Thus, the combination of FMP-mediated, self-regulating hyperthermia with Hsp90 inhibition has important implications for the treatment of cancer.
Figures
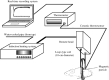
 ) and rectal (
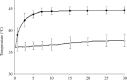
) and rectal ( ) temperatures were measured using a ceramic thermocouple. Symbols represent mean ± SD of five mice.
) temperatures were measured using a ceramic thermocouple. Symbols represent mean ± SD of five mice.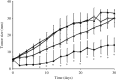
 ; control, n = 10), II (
; control, n = 10), II ( ; one hyperthermia treatment, n = 10), III (
; one hyperthermia treatment, n = 10), III ( ; geldanamycin treatment alone, n = 10), and IV (
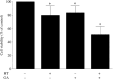
; geldanamycin treatment alone, n = 10), and IV ( ; geldanamycin + hyperthermia, n = 9). Symbols depict mean ± SD. *P < 0.05 versus group I (control).
; geldanamycin + hyperthermia, n = 9). Symbols depict mean ± SD. *P < 0.05 versus group I (control).
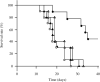
 , group I (n = 10);
, group I (n = 10);  , group II (n = 10);
, group II (n = 10);  , group III (n = 10); and
, group III (n = 10); and  , group IV (n = 9). Survival in group IV was significantly prolonged compared to the other groups (P < 0.05 versus all other groups).
, group IV (n = 9). Survival in group IV was significantly prolonged compared to the other groups (P < 0.05 versus all other groups).References
-
- Cavaliere R, Ciocatto EC, Giovanella BC et al . Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 1967; 20: 1351–81. - PubMed
-
- Overgaard K, Overgaard J. Investigations on the possibility of a thermic tumour therapy. I. Short‐wave treatment of a transplanted isologous mouse mammary carcinoma. Eur J Cancer 1972; 8: 65–78. - PubMed
-
- Lefor AT, Makohon S, Ackerman NB. The effects of hyperthermia on vascular permeability in experimental liver metastasis. J Surg Oncol 1985; 28: 297–300. - PubMed
-
- Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, Abe M. Radiofrequency capacitive hyperthermia for deep‐seated tumors. I. Studies on thermometry. Cancer 1987; 60: 121–7. - PubMed
-
- Van Der Zee J. Heating the patient: a promising approach? Ann Oncol 2002; 13: 1173–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

