Accelerated inactivation of cardiac L-type calcium channels triggered by anaesthetic-induced preconditioning
- PMID: 19154423
- PMCID: PMC2697672
- DOI: 10.1111/j.1476-5381.2008.00026.x
Accelerated inactivation of cardiac L-type calcium channels triggered by anaesthetic-induced preconditioning
Abstract
Background and purpose: Cardioprotection against ischaemia by anaesthetic-induced preconditioning (APC) is well established. However, the mechanism underlying Ca(2+) overload attenuation by APC is unknown. The effects of APC by isoflurane on the cardiac L-type Ca channel were investigated.
Experimental approach: In a model of in vivo APC, Wistar rats were exposed to isoflurane (1.4%), delivered via a vaporizer in an enclosure, prior to thoracotomy. The Dahl S rats were similarly preconditioned to determine strain-dependent effects. Whole-cell patch clamp using cardiac ventricular myocytes was used to determine the L-type Ca(2+) current (I(Ca,L)) characteristics and calmodulin (CaM) levels were determined by Western blot analysis. Cytosolic Ca(2+) levels were monitored using fluo-4-AM. Action potential (AP) simulations examined the effects of APC.
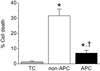
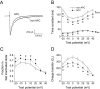
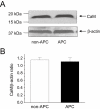
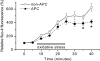
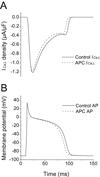
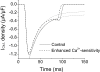
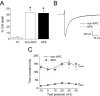
Key results: In Wistar rats, APC significantly accelerated I(Ca,L) inactivation kinetics. This was abolished when external Ca(2+) was replaced with Ba(2+), suggesting that Ca(2+)-dependent inactivation of I(Ca,L) was modulated by APC. Expression levels of CaM, a determinant of I(Ca,L) inactivation, were not affected. Attenuation of cytosolic Ca(2+) accumulation following oxidative stress was observed in the APC group. Simulations showed that the accelerated inactivation of I(Ca,L) resulted in a shortening of the AP duration. The Dahl S rat strain was resistant to APC and changes in I(Ca,L) inactivation were not observed in cardiomyocytes prepared from these rats.
Conclusions and implications: APC triggered persistent changes in the inactivation of cardiac L-type Ca channels. This can potentially lead to a reduction in Ca(2+) influx and attenuation of Ca(2+) overload during ischaemia/reperfusion.
Figures

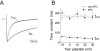
Similar articles
-
Sevoflurane protects ventricular myocytes against oxidative stress-induced cellular Ca2+ overload and hypercontracture.Anesthesiology. 2013 Sep;119(3):606-20. doi: 10.1097/ALN.0b013e318292ee52. Anesthesiology. 2013. PMID: 23571639
-
Impact of in vivo preconditioning by isoflurane on adenosine triphosphate-sensitive potassium channels in the rat heart: lasting modulation of nucleotide sensitivity during early memory period.Anesthesiology. 2006 Mar;104(3):503-10. doi: 10.1097/00000542-200603000-00018. Anesthesiology. 2006. PMID: 16508398
-
Pharmacological preconditioning by diazoxide downregulates cardiac L-type Ca(2+) channels.Br J Pharmacol. 2010 Nov;161(5):1172-85. doi: 10.1111/j.1476-5381.2010.00960.x. Br J Pharmacol. 2010. PMID: 20636393 Free PMC article.
-
Isoflurane protects cardiomyocytes and mitochondria by immediate and cytosol-independent action at reperfusion.Br J Pharmacol. 2010 May;160(2):220-32. doi: 10.1111/j.1476-5381.2010.00698.x. Br J Pharmacol. 2010. PMID: 20423337 Free PMC article.
-
Quantification of calcium entry at the T-tubules and surface membrane in rat ventricular myocytes.Biophys J. 2006 Jan 1;90(1):381-9. doi: 10.1529/biophysj.105.069013. Epub 2005 Oct 7. Biophys J. 2006. PMID: 16214862 Free PMC article.
Cited by
-
New and revisited approaches to preserving the reperfused myocardium.Nat Rev Cardiol. 2017 Nov;14(11):679-693. doi: 10.1038/nrcardio.2017.102. Epub 2017 Jul 27. Nat Rev Cardiol. 2017. PMID: 28748958 Free PMC article. Review.
-
Mitochondrial unfolded protein response, mitophagy and other mitochondrial quality control mechanisms in heart disease and aged heart.Croat Med J. 2020 Apr 30;61(2):126-138. doi: 10.3325/cmj.2020.61.126. Croat Med J. 2020. PMID: 32378379 Free PMC article. Review.
-
Subcellular Energetics and Metabolism: Potential Therapeutic Applications.Anesth Analg. 2017 Jun;124(6):1872-1885. doi: 10.1213/ANE.0000000000001865. Anesth Analg. 2017. PMID: 28277320 Free PMC article. Review.
-
Partial restoration of the long QT syndrome associated KCNQ1 A341V mutant by the KCNE1 β-subunit.Biochim Biophys Acta. 2011 Dec;1810(12):1285-93. doi: 10.1016/j.bbagen.2011.07.018. Epub 2011 Aug 10. Biochim Biophys Acta. 2011. PMID: 21854832 Free PMC article.
-
Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+.Am J Physiol Cell Physiol. 2010 Aug;299(2):C506-15. doi: 10.1152/ajpcell.00006.2010. Epub 2010 Jun 2. Am J Physiol Cell Physiol. 2010. PMID: 20519447 Free PMC article.
References
-
- Aizawa K, Turner LA, Weihrauch D, Bosnjak ZJ, Kwok WM. Protein kinase C-epsilon primes the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel to modulation by isoflurane. Anesthesiology. 2004;101:381–389. - PubMed
-
- An J, Varadarajan SG, Novalija E, Stowe DF. Ischemic and anesthetic preconditioning reduces cytosolic [Ca2+] and improves Ca(2+) responses in intact hearts. Am J Physiol Heart Circ Physiol. 2001;281:H1508–H1523. - PubMed
-
- Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ. Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am J Physiol Heart Circ Physiol. 2000;278:H1395–H1400. - PubMed
-
- Bienengraeber MW, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics. Vascul Pharmacol. 2005;42:243–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

