Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons
- PMID: 19154428
- PMCID: PMC2697673
- DOI: 10.1111/j.1476-5381.2008.00058.x
Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons
Abstract
Background and purpose: The relative contribution of distinct ecto-nucleotidases to the modulation of purinergic signalling may depend on differential tissue distribution and substrate preference.
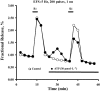
Experimental approach: Extracellular ATP catabolism (assessed by high-performance liquid chromatography) and its influence on [(3)H]acetylcholine ([(3)H]ACh) release were investigated in the myenteric plexus of rat ileum in vitro.
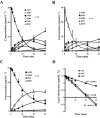
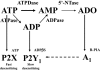
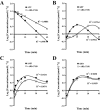
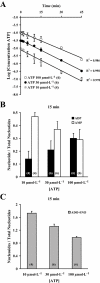
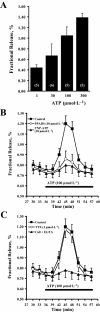
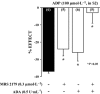
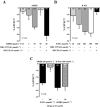
Key results: ATP was primarily metabolized via ecto-ATPDase (adenosine 5'-triphosphate diphosphohydrolase) into AMP, which was then dephosphorylated into adenosine by ecto-5'-nucleotidase. Alternative conversion of ATP into ADP by ecto-ATPase (adenosine 5'-triphosphatase) was more relevant at high ATP concentrations. ATP transiently increased basal [(3)H]ACh outflow in a 2',3'-O-(2,4,6-trinitrophenyl)adenosine-5'-triphosphate (TNP-ATP)-dependent, tetrodotoxin-independent manner. ATP and ATPgammaS (adenosine 5'-[gamma-thio]triphosphate), but not alpha,beta-methyleneATP, decreased [(3)H]ACh release induced by electrical stimulation. ADP and ADPbetaS (adenosine 5'[beta-thio]diphosphate) only decreased evoked [(3)H]ACh release. Inhibition by ADPbetaS was prevented by MRS 2179 (2'-deoxy-N(6)-methyl adenosine 3',5'-diphosphate diammonium salt, a selective P2Y(1) antagonist); blockade of ADP inhibition required co-application of MRS 2179 plus adenosine deaminase (which inactivates endogenous adenosine). Blockade of adenosine A(1) receptors with 1,3-dipropyl-8-cyclopentyl xanthine enhanced ADPbetaS inhibition, indicating that P2Y(1) stimulation is cut short by tonic adenosine A(1) receptor activation. MRS 2179 facilitated evoked [(3)H]ACh release, an effect reversed by the ecto-ATPase inhibitor, ARL67156, which delayed ATP conversion into ADP without affecting adenosine levels.
Conclusions and implications: ATP transiently facilitated [(3)H]ACh release from non-stimulated nerve terminals via prejunctional P2X (probably P2X(2)) receptors. Hydrolysis of ATP directly into AMP by ecto-ATPDase and subsequent formation of adenosine by ecto-5'-nucleotidase reduced [(3)H]ACh release via inhibitory adenosine A(1) receptors. Stimulation of inhibitory P2Y(1) receptors by ADP generated alternatively via ecto-ATPase might be relevant in restraining ACh exocytosis when ATP saturates ecto-ATPDase activity.
Figures
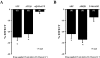
Similar articles
-
A1-receptor-mediated effect of adenosine on the release of acetylcholine from the myenteric plexus: role and localization of ecto-ATPase and 5'-nucleotidase.Neuroscience. 1995 Jul;67(1):159-68. doi: 10.1016/0306-4522(94)00585-s. Neuroscience. 1995. PMID: 7477896
-
Dual effects of adenosine on acetylcholine release from myenteric motoneurons are mediated by junctional facilitatory A(2A) and extrajunctional inhibitory A(1) receptors.Br J Pharmacol. 2004 Mar;141(6):925-34. doi: 10.1038/sj.bjp.0705697. Epub 2004 Mar 1. Br J Pharmacol. 2004. PMID: 14993098 Free PMC article.
-
The pharmacology and kinetics of ecto-nucleotidases in the perilymphatic compartment of the guinea-pig cochlea.Hear Res. 1998 Mar;117(1-2):71-80. doi: 10.1016/s0378-5955(98)00004-5. Hear Res. 1998. PMID: 9580435
-
Purinergic regulation of acetylcholine release.Prog Brain Res. 1996;109:231-41. doi: 10.1016/s0079-6123(08)62107-x. Prog Brain Res. 1996. PMID: 9009712 Review.
-
Extracellular ATP metabolism on vascular endothelial cells: A pathway with pro-thrombotic and anti-thrombotic molecules.Vascul Pharmacol. 2015 Dec;75:1-6. doi: 10.1016/j.vph.2015.05.002. Epub 2015 May 16. Vascul Pharmacol. 2015. PMID: 25989108 Review.
Cited by
-
Purinergic signalling in the gastrointestinal tract and related organs in health and disease.Purinergic Signal. 2014 Mar;10(1):3-50. doi: 10.1007/s11302-013-9397-9. Epub 2013 Dec 4. Purinergic Signal. 2014. PMID: 24307520 Free PMC article. Review.
-
P2Y receptor-mediated transient relaxation of rat longitudinal ileum preparations involves phospholipase C activation, intracellular Ca(2+) release and SK channel activation.Acta Pharmacol Sin. 2016 May;37(5):617-28. doi: 10.1038/aps.2015.137. Epub 2016 Mar 28. Acta Pharmacol Sin. 2016. PMID: 27018177 Free PMC article.
-
Adenosine: Direct and Indirect Actions on Gastric Acid Secretion.Front Physiol. 2017 Sep 22;8:737. doi: 10.3389/fphys.2017.00737. eCollection 2017. Front Physiol. 2017. PMID: 29018360 Free PMC article. Review.
-
P2Y Receptors Sensitize Mouse and Human Colonic Nociceptors.J Neurosci. 2016 Feb 24;36(8):2364-76. doi: 10.1523/JNEUROSCI.3369-15.2016. J Neurosci. 2016. PMID: 26911685 Free PMC article.
-
The Effect of Ischemia and Reperfusion on Enteric Glial Cells and Contractile Activity in the Ileum.Dig Dis Sci. 2015 Sep;60(9):2677-89. doi: 10.1007/s10620-015-3663-3. Epub 2015 Apr 28. Dig Dis Sci. 2015. PMID: 25917048
References
-
- Barajas-López C, Espinosa-Luna R, Gerzanich V. ATP closes a potassium and opens a cationic conductance through different receptors in neurons of guinea-pig submucous plexus. J Pharmacol Exp Ther. 1994;268:1396–1402. - PubMed
-
- Barthó L, Undi S, Benkó R, Wolf M, Lázár Z, Lénárd L, Jr, et al. Multiple motor effects of ATP and their inhibition by P2 purinoceptor antagonist, pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid in the small intestine of the guine-pig. Basic Clin Pharmacol Toxicol. 2006;98:488–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

