Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice
- PMID: 19154433
- PMCID: PMC2697832
- DOI: 10.1111/j.1476-5381.2008.00006.x
Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice
Abstract
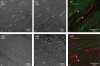
Background and purpose: W/W(v) and wild-type murine bladders were studied to determine whether the W/W(v) phenotype, which causes a reduction in, but not abolition of, tyrosine kinase activity, is a useful tool to study the function of bladder interstitial cells of Cajal (ICC).
Experimental approach: Immunohistochemistry, tension recordings and microelectrode recordings of membrane potential were performed on wild-type and mutant bladders.
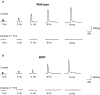
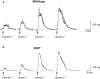
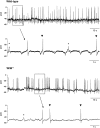
Key results: Wild-type and W/W(v) detrusors contained c-Kit- and vimentin-immunopositive cells in comparable quantities, distribution and morphology. Electrical field stimulation evoked tetrodotoxin-sensitive contractions in wild-type and W/W(v) detrusor strips. Atropine reduced wild-type responses by 50% whereas a 25% reduction occurred in W/W(v) strips. The atropine-insensitive component was blocked by pyridoxal-5-phosphate-6-azophenyl-2',4'-disulphonic acid in both tissue types. Wild-type and W/W(v) detrusors had similar resting membrane potentials of -48 mV. Spontaneous electrical activity in both tissue types comprised action potentials and unitary potentials. Action potentials were nifedipine-sensitive whereas unitary potentials were not. Excitatory junction potentials were evoked by single pulses in both tissues. These were reduced by atropine in wild-type tissues but not in W/W(v) preparations. The atropine-insensitive component was abolished by pyridoxal-5-phosphate-6-azophenyl-2',4'-disulphonic acid in both preparations.
Conclusions and implications: Bladders from W/W(v) mice contain c-Kit- and vimentin-immunopositive ICC. There are similarities in the electrical and contractile properties of W/W(v) and wild-type detrusors. However, significant differences were found in the pharmacology of the responses to neurogenic stimulation with an apparent up-regulation of the purinergic component. These findings indicate that the W/W(v) strain may not be the best model to study ICC function in the bladder.
Figures


Similar articles
-
Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation.J Physiol. 2003 Dec 1;553(Pt 2):545-59. doi: 10.1113/jphysiol.2003.050419. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500772 Free PMC article.
-
Cholinergic neuromuscular transmission mediated by interstitial cells of Cajal in the myenteric layer in mouse ileal longitudinal smooth muscles.Naunyn Schmiedebergs Arch Pharmacol. 2014 Apr;387(4):377-88. doi: 10.1007/s00210-013-0944-2. Epub 2013 Dec 10. Naunyn Schmiedebergs Arch Pharmacol. 2014. PMID: 24322587
-
Neurotransmission in lower esophageal sphincter of W/Wv mutant mice.Am J Physiol Gastrointest Liver Physiol. 2010 Jan;298(1):G14-24. doi: 10.1152/ajpgi.00266.2009. Epub 2009 Oct 22. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19850967
-
Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists.Am J Physiol Renal Physiol. 2007 Mar;292(3):F1065-72. doi: 10.1152/ajprenal.00229.2006. Epub 2006 Nov 14. Am J Physiol Renal Physiol. 2007. PMID: 17107944 Free PMC article.
-
Electromyographic detection of purinergic activity in Guinea pig detrusor smooth muscle.J Urol. 2003 Jan;169(1):377-81. doi: 10.1016/S0022-5347(05)64131-6. J Urol. 2003. PMID: 12478194
Cited by
-
Unique properties of muscularis mucosae smooth muscle in guinea pig urinary bladder.Am J Physiol Regul Integr Comp Physiol. 2011 Aug;301(2):R351-62. doi: 10.1152/ajpregu.00656.2010. Epub 2011 Jun 1. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21632849 Free PMC article.
-
Possible antagonistic effects of the TRPC4 channel blocker ML204 on M2 and M3 muscarinic receptors in mouse ileal and detrusor smooth muscles and atrial myocardium.J Vet Med Sci. 2018 Sep 13;80(9):1407-1415. doi: 10.1292/jvms.18-0197. Epub 2018 Jul 5. J Vet Med Sci. 2018. PMID: 29973432 Free PMC article.
-
Involvement of interstitial cells of Cajal in bladder dysfunction in mice with experimental autoimmune encephalomyelitis.Int Urol Nephrol. 2017 Aug;49(8):1353-1359. doi: 10.1007/s11255-017-1597-8. Epub 2017 Apr 19. Int Urol Nephrol. 2017. PMID: 28425078
-
Platelet-derived growth factor receptor-α cells in mouse urinary bladder: a new class of interstitial cells.J Cell Mol Med. 2012 Apr;16(4):691-700. doi: 10.1111/j.1582-4934.2011.01506.x. J Cell Mol Med. 2012. PMID: 22151424 Free PMC article.
-
Purinergic signalling in the urinary bladder - When function becomes dysfunction.Auton Neurosci. 2021 Nov;235:102852. doi: 10.1016/j.autneu.2021.102852. Epub 2021 Jul 17. Auton Neurosci. 2021. PMID: 34329833 Free PMC article.
References
-
- Banks FC, Knight GE, Calvert RC, Morgan RJ, Burnstock G. Alterations in purinergic and cholinergic components of contractile responses of isolated detrusor contraction in a rat model of partial bladder outlet obstruction. BJU Int. 2006;97:372–378. - PubMed
-
- Bayliss M, Wu C, Newgreen D, Mundy AR, Fry CH. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J Urol. 1999;162:1833–1839. - PubMed
-
- Bernstein A, Chabot B, Dubreuil P, Reith A, Nocka K, Majumder S, et al. The mouse W/c-kit locus. Ciba Found Symp. 1990;148:158–166. 166–172. discussion. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

