Incomplete dissociation of glibenclamide from wild-type and mutant pancreatic K ATP channels limits their recovery from inhibition
- PMID: 19154434
- PMCID: PMC2697833
- DOI: 10.1111/j.1476-5381.2008.00005.x
Incomplete dissociation of glibenclamide from wild-type and mutant pancreatic K ATP channels limits their recovery from inhibition
Abstract
Background and purpose: The antidiabetic sulphonylurea, glibenclamide, acts by inhibiting the pancreatic ATP-sensitive K(+) (K(ATP)) channel, a tetradimeric complex of K(IR)6.2 and sulphonylurea receptor 1 (K(IR)6.2/SUR1)(4). At room temperature, recovery of channel activity following washout of glibenclamide is very slow and cannot be measured. This study investigates the relation between the recovery of channel activity from glibenclamide inhibition and the dissociation rate of [(3)H]-glibenclamide from the channel at 37 degrees C.
Experimental approach: K(IR)6.2, K(IR)6.2DeltaN5 or K(IR)6.2DeltaN10 (the latter lacking amino-terminal residues 2-5 or 2-10 respectively) were coexpressed with SUR1 in HEK cells. Dissociation of [(3)H]-glibenclamide from the channel and recovery of channel activity from glibenclamide inhibition were determined at 37 degrees C.
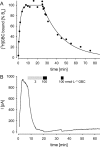
Key results: The dissociation kinetics of [(3)H]-glibenclamide from the wild-type channel followed an exponential decay with a dissociation half-time, t(1/2)(D) = 14 min; however, only limited and slow recovery of channel activity was observed. t(1/2)(D) for K(IR)6.2DeltaN5/SUR1 channels was 5.3 min and recovery of channel activity exhibited a sluggish sigmoidal time course with a half-time, t(1/2)(R) = 12 min. t(1/2)(D) for the DeltaN10 channel was 2.3 min; recovery kinetics were again sigmoidal with t(1/2)(R) approximately 4 min.
Conclusions and implications: The dissociation of glibenclamide from the truncated channels is the rate-limiting step of channel recovery. The sigmoidal recovery kinetics are in quantitative agreement with a model where glibenclamide must dissociate from all four (or at least three) sites before the channel reopens. It is argued that these conclusions hold also for the wild-type (pancreatic) K(ATP) channel.
Figures


References
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzáles G, et al. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- Ashfield R, Gribble FM, Ashcroft SJH, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the KATP channel. Diabetes. 1999;48:1341–1347. - PubMed
-
- Babenko AP. KATP channels ‘vingt ans apres’: ATG to PDB to Mechanism. J Mol Cell Cardiol. 2005;39:79–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

