The Jekyll and Hyde functions of caspases
- PMID: 19154716
- PMCID: PMC2850564
- DOI: 10.1016/j.devcel.2008.12.012
The Jekyll and Hyde functions of caspases
Abstract
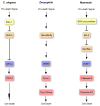
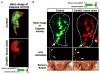
Apoptosis is an ancient form of regulated cell death that functions under pathological and nonpathological contexts in all metazoans. More than a decade of intense research has led to extensive characterization of the core molecular mechanisms for apoptotic cell death. This includes the identification of a family of cysteine proteases, caspases, which are critical for the execution of apoptosis. Whereas completion of the proteolytic caspase cascade leads to elimination of a cell by apoptosis, caspase activation, when finely tuned, directs alternative cellular functions independent of cell death. Exciting recent developments have focused on uncovering nonapoptotic roles of caspases ranging from immune regulation to spermatogenesis, in highly specialized cellular frameworks.
Figures



References
-
- Allan LA, Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell. 2007;26:301–310. - PubMed
-
- Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. - PubMed
-
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. - PubMed
-
- Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

