Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti
- PMID: 19154718
- PMCID: PMC2696015
- DOI: 10.1016/j.devcel.2008.11.008
Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti
Abstract
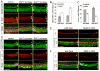
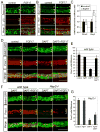
The organ of Corti, the auditory organ of the inner ear, contains two types of sensory hair cells and at least seven types of supporting cells. Most of these supporting cell types rely on Notch-dependent expression of Hes/Hey transcription factors to maintain the supporting cell fate. Here, we show that Notch signaling is not necessary for the differentiation and maintenance of pillar cell fate, that pillar cells are distinguished by Hey2 expression, and that-unlike other Hes/Hey factors-Hey2 expression is Notch independent. Hey2 is activated by FGF and blocks hair cell differentiation, whereas mutation of Hey2 leaves pillar cells sensitive to the loss of Notch signaling and allows them to differentiate as hair cells. We speculate that co-option of FGF signaling to render Hey2 Notch independent also liberated pillar cells from the need for direct contact with surrounding hair cells, and enabled evolutionary remodeling of the complex cellular mosaic of the inner ear.
Figures





References
-
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development (Cambridge, England) 1998;125:4645–4654. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development (Cambridge, England) 2004;131:4119–4130. - PubMed
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

