TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration
- PMID: 19154987
- PMCID: PMC2768384
- DOI: 10.1016/j.chom.2008.11.009
TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration
Abstract
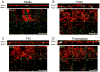
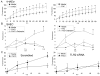
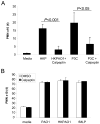
Recruitment of polymorphonuclear leukocytes (PMNs) into the lungs in response to inhaled pathogens is initiated by epithelial signaling, the activation of toll-like receptors (TLRs), and the production of the chemokine interleukin-8. To reach the site of infection, PMNs must be mobilized through epithelial junctions. Here, we demonstrate that Ca(2+) fluxes generated by TLR2 signals activate calpains, Ca(2+)-dependent cysteine proteases. These activated calpains cleave the transmembrane junctional proteins occludin and E-cadherin without breaching the integrity of the epithelial barrier. Calpain inhibitors decrease PMN transepithelial migration in response to TLR2 agonists both in vitro and in a mouse model of P. aeruginosa infection. Thus, TLR2 signaling in the airway not only induces chemokine expression to recruit PMNs, but also initiates cleavage of junctional proteins to accommodate transmigration of the recruited PMNs.
Figures



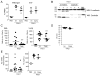
Comment in
-
Epithelium, tear down this wall!Cell Host Microbe. 2009 Jan 22;5(1):1-2. doi: 10.1016/j.chom.2008.12.008. Cell Host Microbe. 2009. PMID: 19154980
Similar articles
-
Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration.J Leukoc Biol. 2009 Nov;86(5):1135-44. doi: 10.1189/jlb.0209072. Epub 2009 Jul 15. J Leukoc Biol. 2009. PMID: 19605699 Free PMC article. Review.
-
Weakened Airway Epithelial Junctions and Enhanced Neutrophil Elastase Release Contribute to Age-Dependent Bacteremia Risk Following Pneumococcal Pneumonia.Aging Cell. 2025 May;24(5):e14474. doi: 10.1111/acel.14474. Epub 2025 Jan 8. Aging Cell. 2025. PMID: 39778043 Free PMC article.
-
Streptolysin S contributes to group A streptococcal translocation across an epithelial barrier.J Biol Chem. 2011 Jan 28;286(4):2750-61. doi: 10.1074/jbc.M110.171504. Epub 2010 Nov 17. J Biol Chem. 2011. PMID: 21084306 Free PMC article.
-
Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5.Am J Respir Cell Mol Biol. 2004 May;30(5):627-34. doi: 10.1165/rcmb.2003-0260OC. Epub 2003 Nov 7. Am J Respir Cell Mol Biol. 2004. PMID: 14607814
-
Vascular and epithelial junctions: a barrier for leucocyte migration.Biochem Soc Trans. 2008 Apr;36(Pt 2):203-11. doi: 10.1042/BST0360203. Biochem Soc Trans. 2008. PMID: 18363562 Review.
Cited by
-
Candida-streptococcal mucosal biofilms display distinct structural and virulence characteristics depending on growth conditions and hyphal morphotypes.Mol Oral Microbiol. 2015 Aug;30(4):307-22. doi: 10.1111/omi.12095. Epub 2015 Apr 20. Mol Oral Microbiol. 2015. PMID: 25754666 Free PMC article.
-
Cleavage of Occludin by Cigarette Smoke-Elicited Cathepsin S Increases Permeability of Lung Epithelial Cells.Antioxidants (Basel). 2022 Dec 21;12(1):5. doi: 10.3390/antiox12010005. Antioxidants (Basel). 2022. PMID: 36670867 Free PMC article.
-
Increased Expression of Tight Junction Proteins and Blood-Brain Barrier Integrity in MCAO Rats Following Injection of miR-149-5p.Int J Mol Cell Med. 2022;11(3):223-235. doi: 10.22088/IJMCM.BUMS.11.3.223. Int J Mol Cell Med. 2022. PMID: 37605737 Free PMC article.
-
Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium.Cell Host Microbe. 2011 May 19;9(5):404-14. doi: 10.1016/j.chom.2011.04.012. Cell Host Microbe. 2011. PMID: 21575911 Free PMC article.
-
Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth.J Cell Mol Med. 2016 Apr;20(4):758-64. doi: 10.1111/jcmm.12784. Epub 2016 Feb 2. J Cell Mol Med. 2016. PMID: 26837005 Free PMC article.
References
-
- Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci. 1999;112(Pt 12):1879–1888. - PubMed
-
- Bojarski C, Weiske J, Schoneberg T, Schroder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117:2097–2107. - PubMed
-
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. - PubMed
-
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci. 2007;120:1818–1828. - PubMed
-
- Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

