Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus
- PMID: 19154988
- PMCID: PMC2642927
- DOI: 10.1016/j.chom.2008.12.001
Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus
Abstract
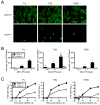
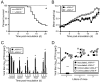
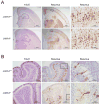
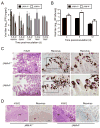
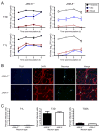
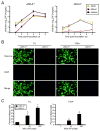
Diverse families of viruses bind immunoglobulin superfamily (IgSF) proteins located in tight junctions (TJs) and adherens junctions of epithelium and endothelium. However, little is known about the roles of these receptors in the pathogenesis of viral disease. Junctional adhesion molecule-A (JAM-A) is an IgSF protein that localizes to TJs and serves as a receptor for mammalian reovirus. We inoculated wild-type (WT) and isogenic JAM-A(-/-) mice perorally with reovirus and found that JAM-A is dispensable for viral replication in the intestine but required for systemic dissemination. Reovirus replication in the brain and tropism for discrete neural regions are equivalent in WT and JAM-A(-/-) mice following intracranial inoculation, suggesting a function for JAM-A in reovirus spread to extraintestinal sites. JAM-A promotes reovirus infection of endothelial cells, providing a conduit for the virus into the bloodstream. These findings indicate that a broadly expressed IgSF viral receptor specifically mediates hematogenous dissemination in the host.
Figures

Comment in
-
The distinct roles of JAM-A in reovirus pathogenesis.Cell Host Microbe. 2009 Jan 22;5(1):3-5. doi: 10.1016/j.chom.2008.12.009. Cell Host Microbe. 2009. PMID: 19154981
References
-
- Barton ES, Connolly JL, Forrest JC, Chappell JD, Dermody TS. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J Biol Chem. 2001a;276:2200–2211. - PubMed
-
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell F, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001b;104:441–451. - PubMed
-
- Bass DM, Trier JS, Dambrauskas R, Wolf JL. Reovirus type 1 infection of small intestinal epithelium in suckling mice and its effect on M cells. Lab Invest. 1988;58:226–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

