FBP17 Mediates a Common Molecular Step in the Formation of Podosomes and Phagocytic Cups in Macrophages
- PMID: 19155218
- PMCID: PMC2659213
- DOI: 10.1074/jbc.M805638200
FBP17 Mediates a Common Molecular Step in the Formation of Podosomes and Phagocytic Cups in Macrophages
Abstract
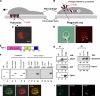
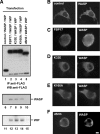
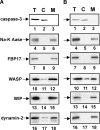
Macrophages act to protect the body against inflammation and infection by engaging in chemotaxis and phagocytosis. In chemotaxis, macrophages use an actin-based membrane structure, the podosome, to migrate to inflamed tissues. In phagocytosis, macrophages form another type of actin-based membrane structure, the phagocytic cup, to ingest foreign materials such as bacteria. The formation of these membrane structures is severely affected in macrophages from patients with Wiskott-Aldrich syndrome (WAS), an X chromosome-linked immunodeficiency disorder. WAS patients lack WAS protein (WASP), suggesting that WASP is required for the formation of podosomes and phagocytic cups. Here we have demonstrated that formin-binding protein 17 (FBP17) recruits WASP, WASP-interacting protein (WIP), and dynamin-2 to the plasma membrane and that this recruitment is necessary for the formation of podosomes and phagocytic cups. The N-terminal EFC (extended FER-CIP4 homology)/F-BAR (FER-CIP4 homology and Bin-amphiphysin-Rvs) domain of FBP17 was previously shown to have membrane binding and deformation activities. Our results suggest that FBP17 facilitates membrane deformation and actin polymerization to occur simultaneously at the same membrane sites, which mediates a common molecular step in the formation of podosomes and phagocytic cups. These results provide a potential mechanism underlying the recurrent infections in WAS patients.
Figures

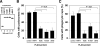
Similar articles
-
Requirement for a complex of Wiskott-Aldrich syndrome protein (WASP) with WASP interacting protein in podosome formation in macrophages.J Immunol. 2007 Mar 1;178(5):2987-95. doi: 10.4049/jimmunol.178.5.2987. J Immunol. 2007. PMID: 17312144 Free PMC article.
-
Wiskott-Aldrich syndrome protein is a key regulator of the phagocytic cup formation in macrophages.J Biol Chem. 2007 Nov 23;282(47):34194-203. doi: 10.1074/jbc.M705999200. Epub 2007 Sep 21. J Biol Chem. 2007. PMID: 17890224
-
Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis.J Cell Biol. 2006 Jan 16;172(2):269-79. doi: 10.1083/jcb.200508091. J Cell Biol. 2006. PMID: 16418535 Free PMC article.
-
Recent advances in the biology of WASP and WIP.Immunol Res. 2009;44(1-3):99-111. doi: 10.1007/s12026-008-8086-1. Immunol Res. 2009. PMID: 19018480 Review.
-
WIP remodeling actin behind the scenes: how WIP reshapes immune and other functions.Int J Mol Sci. 2012;13(6):7629-7647. doi: 10.3390/ijms13067629. Epub 2012 Jun 21. Int J Mol Sci. 2012. PMID: 22837718 Free PMC article. Review.
Cited by
-
Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton.Immunol Rev. 2013 Nov;256(1):222-39. doi: 10.1111/imr.12118. Immunol Rev. 2013. PMID: 24117824 Free PMC article. Review.
-
The F-BAR protein family Actin' on the membrane.Commun Integr Biol. 2010 Mar;3(2):89-94. doi: 10.4161/cib.3.2.10521. Commun Integr Biol. 2010. PMID: 20585497 Free PMC article.
-
Importance of RhoGTPases in formation, characteristics, and functions of invadosomes.Small GTPases. 2014;5:e28195. doi: 10.4161/sgtp.28713. Epub 2014 May 8. Small GTPases. 2014. PMID: 24967648 Free PMC article. Review.
-
Forces and constraints controlling podosome assembly and disassembly.Philos Trans R Soc Lond B Biol Sci. 2019 Aug 19;374(1779):20180228. doi: 10.1098/rstb.2018.0228. Epub 2019 Jul 1. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31431172 Free PMC article.
-
Essential function of dynamin in the invasive properties and actin architecture of v-Src induced podosomes/invadosomes.PLoS One. 2013 Dec 9;8(12):e77956. doi: 10.1371/journal.pone.0077956. eCollection 2013. PLoS One. 2013. PMID: 24348990 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

