The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion
- PMID: 19155290
- PMCID: PMC2714432
- DOI: 10.1242/jcs.037200
The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion
Abstract
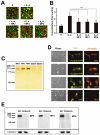
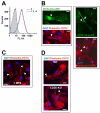
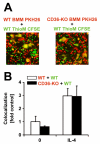
Multinucleated giant cells, characteristic of granulomatous infections, originate from the fusion of macrophages. Using an antibody screening strategy we found that the scavenger receptor CD36 participates in macrophage fusion induced by the cytokines IL-4 and GM-CSF. Our results demonstrate that exposure of phosphatidylserine on the cell surface and lipid recognition by CD36 are required for cytokine-induced fusion of macrophages. We also show that CD36 acts in a heterotypic manner during giant-cell formation and that the formation of osteoclasts is independent of CD36. The discovery of molecules involved in the formation of multinucleated giant cells will enable us to determine their functional significance. Furthermore, our results suggest that lipid capture by cell surface receptors may be a general feature of cell fusion.
Figures



Similar articles
-
Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells.Am J Pathol. 1995 Nov;147(5):1487-99. Am J Pathol. 1995. PMID: 7485411 Free PMC article.
-
Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36.J Biol Chem. 1997 Aug 22;272(34):21654-9. doi: 10.1074/jbc.272.34.21654. J Biol Chem. 1997. PMID: 9261189
-
Mechanotransduction via a TRPV4-Rac1 signaling axis plays a role in multinucleated giant cell formation.J Biol Chem. 2021 Jan-Jun;296:100129. doi: 10.1074/jbc.RA120.014597. Epub 2020 Dec 4. J Biol Chem. 2021. PMID: 33262217 Free PMC article.
-
Macrophage fusion and multinucleated giant cells of inflammation.Adv Exp Med Biol. 2011;713:97-111. doi: 10.1007/978-94-007-0763-4_7. Adv Exp Med Biol. 2011. PMID: 21432016 Review.
-
Monocyte-Macrophage Lineage Cell Fusion.Int J Mol Sci. 2022 Jun 12;23(12):6553. doi: 10.3390/ijms23126553. Int J Mol Sci. 2022. PMID: 35742997 Free PMC article. Review.
Cited by
-
The Morphology and Phenotype of Monocyte-Macrophages When Cultured on Bionanofilms Substrates with Different Surface Relief Profiles.Biomolecules. 2019 Dec 30;10(1):65. doi: 10.3390/biom10010065. Biomolecules. 2019. PMID: 31906038 Free PMC article.
-
Generation of Cancer Stem/Initiating Cells by Cell-Cell Fusion.Int J Mol Sci. 2022 Apr 19;23(9):4514. doi: 10.3390/ijms23094514. Int J Mol Sci. 2022. PMID: 35562905 Free PMC article. Review.
-
Regulation of phospholipid distribution in the lipid bilayer by flippases and scramblases.Nat Rev Mol Cell Biol. 2023 Aug;24(8):576-596. doi: 10.1038/s41580-023-00604-z. Epub 2023 Apr 27. Nat Rev Mol Cell Biol. 2023. PMID: 37106071 Free PMC article. Review.
-
Scavenger receptor CD36 governs recruitment of myeloid cells to the blood-CSF barrier after stroke in neonatal mice.J Neuroinflammation. 2022 Feb 11;19(1):47. doi: 10.1186/s12974-022-02388-z. J Neuroinflammation. 2022. PMID: 35148760 Free PMC article.
-
Low human and murine Mcl-1 expression leads to a pro-apoptotic plaque phenotype enriched in giant-cells.Sci Rep. 2019 Oct 10;9(1):14547. doi: 10.1038/s41598-019-51020-3. Sci Rep. 2019. PMID: 31601924 Free PMC article.
References
-
- Adler, R. R., Ng, A. K. and Rote, N. S. (1995). Monoclonal antiphosphatidylserine antibody inhibits intercellular fusion of the choriocarcinoma line, JAR. Biol. Reprod. 53, 905-910. - PubMed
-
- Anderson, J. M. (2000). Multinucleated giant cells. Curr. Opin. Hematol. 7, 40-47. - PubMed
-
- Byrd, T. F. (1998). Multinucleated giant cell formation induced by IFN-gamma/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocyte monolayers. Cell Immunol. 188, 89-96. - PubMed
-
- Callahan, M. K., Williamson, P. and Schlegel, R. A. (2000). Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 7, 645-653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

