Senescent stromal-derived osteopontin promotes preneoplastic cell growth
- PMID: 19155301
- PMCID: PMC2633422
- DOI: 10.1158/0008-5472.CAN-08-2970
Senescent stromal-derived osteopontin promotes preneoplastic cell growth
Abstract
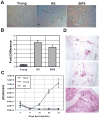
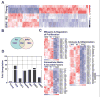
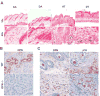
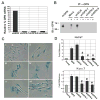
Alterations in the tissue microenvironment collaborate with cell autonomous genetic changes to contribute to neoplastic progression. The importance of the microenvironment in neoplastic progression is underscored by studies showing that fibroblasts isolated from a tumor stimulate the growth of preneoplastic and neoplastic cells in xenograft models. Similarly, senescent fibroblasts promote preneoplastic cell growth in vitro and in vivo. Because senescent cells accumulate with age, their presence is hypothesized to facilitate preneoplastic cell growth and tumor formation in older individuals. To identify senescent stromal factors directly responsible for stimulating preneoplastic cell growth, we carried out whole-genome transcriptional profiling and compared senescent fibroblasts with their younger counterparts. We identified osteopontin (OPN) as one of the most highly elevated transcripts in senescent fibroblasts. Importantly, reduction of OPN protein levels by RNA interference did not affect senescence induction in fibroblasts; however, it dramatically reduced the growth-promoting activities of senescent fibroblasts in vitro and in vivo, showing that OPN is necessary for paracrine stimulation of preneoplastic cell growth. In addition, we found that recombinant OPN was sufficient to stimulate preneoplastic cell growth. Finally, we show that OPN is expressed in senescent stroma within preneoplastic lesions that arise following 7,12-dimethylbenz(a)anthracene/12-O-tetradecanoylphorbol-13-acetate treatment of mice, suggesting that stromal-derived OPN-mediated signaling events affect neoplastic progression.
Figures

References
-
- DePinho RA. The age of cancer. Nature. 2000;408(6809):248–54. - PubMed
-
- Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2 Pt 1):379–85. - PubMed
-
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumor cells with defined genetic elements. Nature. 1999;400:464–8. - PubMed
-
- Hahn WC, Meyerson M. Telomerase activation, cellular immortalization and cancer. Ann Med. 2001;33(2):123–9. - PubMed
-
- Zhao JJ, Roberts TM, Hahn WC. Functional genetics and experimental models of human cancer. Trends Mol Med. 2004;10(7):344–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

