Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial
- PMID: 19155456
- PMCID: PMC2840403
- DOI: 10.1001/jama.2008.977
Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial
Abstract
Context: Generalized anxiety disorder (GAD) is one of the most common psychiatric disorders in older adults; however, few data exist to guide clinicians in efficacious and safe treatment. Selective serotonin reuptake inhibitors (SSRIs) are efficacious for younger adults with GAD, but benefits and risks may be different in older adults.
Objective: To examine the efficacy, safety, and tolerability of the SSRI escitalopram in older adults with GAD.
Design, setting, and participants: A randomized controlled trial in primary care practices and related specialty clinics in Pittsburgh, Pennsylvania, of 177 participants aged 60 years or older with a principal diagnosis of GAD randomized to receive either escitalopram or placebo and conducted between January 2005 and January 2008.
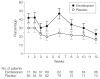
Interventions: Twelve weeks of 10 to 20 mg/d of escitalopram (n = 85) or matching placebo (n = 92).
Main outcome measures: Cumulative response defined by Clinical Global Impressions-Improvement score of much or very much improved; time to response; and anxiety and role functioning changes measured by the Clinical Global Impressions-Improvement scale, Hamilton Anxiety Rating Scale, Penn State Worry Questionnaire, Late-Life Function and Disability Instrument activity limitations subscale, and the role-emotional impairment and social function subscales of the Medical Outcome Survey 36-item Short Form.
Results: In the primary analytic strategy in which participants (n = 33) were censored at the time of dropout, mean cumulative response rate for escitalopram was 69% (95% confidence interval [CI], 58%-80%) vs 51% (95% CI, 40%-62%) for placebo (P = .03). A conservative intention-to-treat analysis showed no difference in mean cumulative response rate between escitalopram and placebo (57%; 95% CI, 46%-67%; vs 45%; 95% CI, 35%-55%; P = .11). Participants treated with escitalopram showed greater improvement than with placebo in anxiety symptoms and role functioning (Clinical Global Impressions-Improvement scale: effect size, 0.93; 95% CI, 0.50-1.36; P < .001; Penn State Worry Questionnaire: 0.30; 95% CI, 0.23-0.48; P = .01; activity limitations: 0.32; 95% CI, 0.01-0.63; P = .04; and the role-emotional impairment and social function: 0.96; 95% CI, 0.03-1.90; P = .04). Adverse effects of escitalopram (P < .05 vs placebo) were fatigue or somnolence (35 patients [41.1%]), sleep disturbance (12 [14.1%]), and urinary symptoms (8 [9.4%]).
Conclusions: Older adults with GAD randomized to escitalopram had a higher cumulative response rate for improvement vs placebo over 12 weeks; however, response rates were not significantly different using an intention-to-treat analysis. Further study is required to assess efficacy and safety over longer treatment durations.
Trial registration: clinicaltrials.gov Identifier: NCT00105586.
Figures


Comment in
-
Escitalopram treatment of generalized anxiety disorder in older adults.JAMA. 2009 May 20;301(19):1987-8; author reply 1988. doi: 10.1001/jama.2009.651. JAMA. 2009. PMID: 19454633 No abstract available.
-
Escitalopram modestly improves generalised anxiety disorder in older adults in treatment completers.Evid Based Ment Health. 2009 Aug;12(3):87. doi: 10.1136/ebmh.12.3.87. Evid Based Ment Health. 2009. PMID: 19633255 No abstract available.
References
-
- Ormel J, VonKorff M, Ustun TB, Pini S, Korten A, Oldehinkel T. Common mental disorders and disability across cultures: results from the WHO Collaborative Study on Psychological Problems in General Health Care. JAMA. 1994;272(22):1741–1748. - PubMed
-
- Wittchen HU, Kessler RC, Beesdo K, Krause P, Höfler M, Hoyer J. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63(suppl 8):24–34. - PubMed
-
- Hoffman DL, Dukes EM, Wittchen H-U. Human and economic burden of generalized anxiety disorder. Depress Anxiety. 2008;25(1):72–90. - PubMed
-
- Kessler RC, Keller MB, Wittchen HU. The epidemiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24(1):19–39. - PubMed

