MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes
- PMID: 19155478
- PMCID: PMC2652126
- DOI: 10.4049/jimmunol.182.3.1325
MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes
Abstract
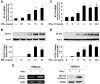
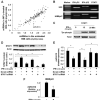
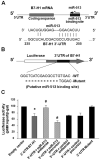
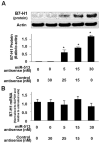
Biliary epithelial cells (cholangiocytes) respond to proinflammatory cytokines such as IFN-gamma and actively participate in the regulation of biliary inflammatory response in the liver. B7-H1 (also known as CD274 or PD-L1) is a member of the B7 costimulatory molecules and plays a critical immunoregulatory role in cell-mediated immune responses. In this study, we show that resting human cholangiocytes in culture express B7-H1 mRNA, but not B7-H1 protein. IFN-gamma induces B7-H1 protein expression and alters the microRNA (miRNA) expression profile in cholangiocytes. Of those IFN-gamma-down-regulated miRNAs, we identified microRNA-513 (miR-513) with complementarity to the 3'-untranslated region of B7-H1 mRNA. Targeting of the B7-H1 3'-untranslated region by miR-513 results in translational repression. Transfection of cholangiocytes with an antisense oligonucleotide to miR-513 induces B7-H1 protein expression. Additionally, transfection of miR-513 precursor decreases IFN-gamma-induced B7-H1 protein expression and consequently influences B7-H1-associated apoptotic cell death in cocultured Jurkat cells. Thus, miR-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in human cholangiocytes, suggesting a role for miRNA-mediated gene silencing in the regulation of cholangiocyte response to IFN-gamma.
Figures

References
-
- Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. - PubMed
-
- Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J. Clin. Gastroenterol. 2005;39:S90–S102. - PubMed
-
- Alpini G, McGill JM, LaRusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. - PubMed
-
- Dong H, Strome S, Salomao DR, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. - PubMed
-
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

