TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses
- PMID: 19155513
- PMCID: PMC3195412
- DOI: 10.4049/jimmunol.182.3.1641
TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses
Abstract
Thymic stromal lymphopoietin (TSLP) is crucial for the development of atopic diseases in humans and mice. Mice that express a lung-specific TSLP transgene (surfactant protein C promoter (SPC)-TSLP) develop a spontaneous and progressive asthma-like disease, suggesting that TSLP expression alone was sufficient for disease development. In this study, we show that, in fact, TSLP alone only causes a weak innate response that is insufficient for development of full airway inflammatory disease. Complete disease development requires both TSLP and antigenic stimulation. These data suggest that the spontaneous lung inflammation observed in SPC-TSLP mice reflects a TSLP-driven predisposition toward the development of aberrant responses against innocuous environmental Ags. This provides evidence that TSLP may act directly to induce susceptibility to the inappropriate allergic responses that characterize atopy and asthma. We additionally show that disease development requires CD4 T cells but not B cells. Further, we reveal a TSLP-driven innate response involving mucus overproduction and goblet cell metaplasia. Taken together, these data suggest a multifaceted model of TSLP-mediated airway inflammation, with an initial activation of resident innate immune cells, followed by activation of the adaptive immune system and full disease development. This study provides new insight into the unique features of the asthma pathology contributed by the innate and adaptive immune responses in response to TSLP stimulation.
Figures





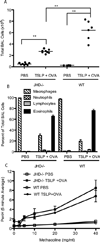
References
-
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annual review of immunology. 2004;22:789–815. - PubMed
-
- Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. - PubMed
-
- Brightling CE, Bradding P. The re-emergence of the mast cell as a pivotal cell in asthma pathogenesis. Current allergy and asthma reports. 2005;5:130–135. - PubMed
-
- Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nature reviews. 2008;8:193–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

