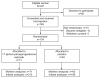
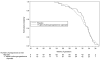
Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial
- PMID: 19155896
- PMCID: PMC2790283
- DOI: 10.1097/AOG.0b013e318193c677
Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial
Abstract
Objective: To assess whether 17 alpha-hydroxyprogesterone caproate reduces the rate of preterm birth in women carrying triplets.
Methods: We performed this randomized, double-blinded, placebo-controlled trial in 14 centers. Healthy women with triplets were randomly assigned to weekly intramuscular injections of either 250 mg of 17 alpha-hydroxyprogesterone caproate or matching placebo, starting at 16-20 weeks and ending at delivery or 35 weeks of gestation. The primary study outcome was delivery or fetal loss before 35 weeks.
Results: One hundred thirty-four women were assigned, 71 to 17 alpha-hydroxyprogesterone caproate and 63 to placebo; none were lost to follow-up. Baseline demographic data were similar in the two groups. The proportion of women experiencing the primary outcome (a composite of delivery or fetal loss before 35 0/7 weeks) was similar in the two treatment groups: 83% of pregnancies in the 17 alpha-hydroxyprogesterone caproate group and 84% in the placebo group, relative risk 1.0, 95% confidence interval 0.9-1.1. The lack of benefit of 17 alpha-hydroxyprogesterone caproate was evident regardless of the conception method or whether a gestational age cutoff for delivery was set at 32 or 28 weeks.
Conclusion: Treatment with 17 alpha-hydroxyprogesterone caproate did not reduce the rate of preterm birth in women with triplet gestations.
Clinical trial registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00099164
Level of evidence: I.
Figures
References
-
- Alexander GR, Salihu HM. Perinatal outcomes of singleton and multiple births in the United States, 1995-98. In: Blickstein I, Keith G, editors. Multiple Pregnancy. Taylor and Francis; London and New York: 2005. pp. 3–10.
-
- Mathews TJ, MacDorman MF. National vital statistics reports. 16. Vol. 54. Hyattsville, MD: National Center for Health Statistics; 2006. Infant mortality statistics from the 2003 period linked birth/infant death data set. - PubMed
-
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of Recurrent Preterm Delivery by 17 Alpha-Hydroxyprogesterone Caproate. N Engl J Med. 2003;348:2379–85. - PubMed
-
- Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–61. - PubMed
-
- Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342:534–540. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- R24 HD050924/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- HD53097/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials