Multi-modality mediastinal staging for lung cancer among medicare beneficiaries
- PMID: 19156000
- PMCID: PMC2726111
- DOI: 10.1097/JTO.0b013e318197f4d9
Multi-modality mediastinal staging for lung cancer among medicare beneficiaries
Abstract
Introduction: The use of noninvasive and invasive diagnostic tests improves the accuracy of mediastinal staging for lung cancer. It is unknown how frequently multimodality mediastinal staging is used, or whether its use is associated with better health outcomes.
Methods: A cohort study was conducted using Surveillance, Epidemiology, and End Results-Medicare data (1998-2005). Patients were categorized as having undergone single (computed tomography [CT] only), bi- (CT and positron emission tomography or CT and invasive staging), or tri-modality (CT, positron emission tomography, and invasive staging) staging.
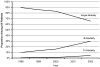
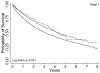
Results: Among 43,912 subjects, 77%, 21%, and 2% received single, bi-, and tri-modality staging, respectively. The use of single modality staging decreased over time from 90% in 1998 to 67% in 2002 (p-trend <0.001), whereas the use of bi- and tri-modality staging increased from 10% to 30% and 0.4% to 5%, respectively. After adjustment for differences in patient characteristics, the use of a greater number of staging modalities was associated with a lower risk of death (bi- versus single modality: hazard ratio [HR] 0.58, 99% confidence interval [CI] 0.56-0.60; tri- versus single modality: HR 0.49, 99% CI 0.45-0.54; tri- versus bi-modality: HR 0.85, 99% CI 0.77-0.93). These associations were maintained even after excluding stage IV patients or adjustment for stage.
Conclusions: The use of multimodality mediastinal staging increased over time and was associated with better survival. Stage migration and unmeasured patient and provider characteristics may have affected the magnitude of these associations. Cancer treatment guidelines should emphasize the potential relationship between staging procedures and outcomes, and health care policy should encourage adherence to staging guidelines.
Figures



Comment in
-
What is quality and does it matter?J Thorac Oncol. 2009 Mar;4(3):279-80. doi: 10.1097/JTO.0b013e3181989be5. J Thorac Oncol. 2009. PMID: 19247081 No abstract available.
References
-
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):202S–220S. - PubMed
-
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):178S–201S. - PubMed
-
- Annema JT, Versteegh MI, Veselic M, et al. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. Jama. 2005;294(8):931–936. - PubMed
-
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348(25):2500–2507. - PubMed
-
- Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343(4):254–261. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

