Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus
- PMID: 19156211
- PMCID: PMC2626279
- DOI: 10.1371/journal.pone.0004235
Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus
Abstract
Background: Although disease remission can frequently be achieved for patients with neuroblastoma, relapse is common. The cancer stem cell theory suggests that rare tumorigenic cells, resistant to conventional therapy, are responsible for relapse. If true for neuroblastoma, improved cure rates may only be achieved via identification and therapeutic targeting of the neuroblastoma tumor initiating cell. Based on cues from normal stem cells, evidence for tumor populating progenitor cells has been found in a variety of cancers.
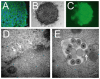
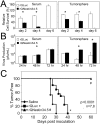
Methodology/principal findings: Four of eight human neuroblastoma cell lines formed tumorspheres in neural stem cell media, and all contained some cells that expressed neurogenic stem cell markers including CD133, ABCG2, and nestin. Three lines tested could be induced into multi-lineage differentiation. LA-N-5 spheres were further studied and showed a verapamil-sensitive side population, relative resistance to doxorubicin, and CD133+ cells showed increased sphere formation and tumorigenicity. Oncolytic viruses, engineered to be clinically safe by genetic mutation, are emerging as next generation anticancer therapeutics. Because oncolytic viruses circumvent typical drug-resistance mechanisms, they may represent an effective therapy for chemotherapy-resistant tumor initiating cells. A Nestin-targeted oncolytic herpes simplex virus efficiently replicated within and killed neuroblastoma tumor initiating cells preventing their ability to form tumors in athymic nude mice.
Conclusions/significance: These results suggest that human neuroblastoma contains tumor initiating cells that may be effectively targeted by an oncolytic virus.
Conflict of interest statement
Figures






References
-
- Olshan A, Bunin G. Epidemiology of Neuroblastoma. In: Brodeur G, Sawada T, Tsuchida Y, Voute P, editors. Neuroblastoma. Amsterdam: Elsevier Science; 2000. pp. 33–39.
-
- Brodeur G, Maris J. Neuroblastoma. In: Pizzo P, Poplack D, editors. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 896–937.
-
- George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. - PubMed
-
- Matthay K, Villablanca J, Seeger R, Stram D, Harris R, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. New England Journal of Medicine. 1999;341:1165–1173. - PubMed
-
- Luo L, Han ZC. Leukemia stem cells. Int J Hematol. 2006;84:123–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

