Heparin antagonism by polyvalent display of cationic motifs on virus-like particles
- PMID: 19156786
- PMCID: PMC2751660
- DOI: 10.1002/cbic.200800493
Heparin antagonism by polyvalent display of cationic motifs on virus-like particles
Abstract
Particles to the rescue! The construction of cationic amino acid motifs on the surface of bacteriophage Qbeta by genetic engineering or chemical conjugation gives particles that are potent inhibitors of the anticoagulant action of heparin, which is a common anticlotting agent subject to clinical overdose.Polyvalent interactions allow biological structures to exploit low-affinity ligand-receptor binding events to affect physiological responses. We describe here the use of bacteriophage Qbeta as a multivalent platform for the display of polycationic motifs that act as heparin antagonists. Point mutations to the coat protein allowed us to generate capsids bearing the K16M, T18R, N10R, or D14R mutations; because 180 coat proteins form the capsid, the mutants provide a spectrum of particles differing in surface charge by as much as +540 units (K16M vs. D14R). Whereas larger poly-Arg insertions (for example, C-terminal Arg(8)) did not yield intact virions, it was possible to append chemically synthesized oligo-Arg peptides to stable wild-type (WT) and K16M platforms. Heparin antagonism by the particles was evaluated by using the activated partial thrombin time (aPTT) clotting assay; this revealed that T18R, D14R, and WT-(R(8)G(2))(95) were the most effective at disrupting heparin-mediated anticoagulation (>95 % inhibition). This activity agreed with measurements of zeta potential (ZP) and retention time on cation exchange chromatography for the genetic constructs, which distribute their added positive charge over the capsid surface (+180 and +360 for T18R and D14R relative to WT). The potent activity of WT-(R(8)G(2))(95), despite its relatively diminished overall surface charge is likely a consequence of the particle's presentation of locally concentrated regions with high positive charge density that interact with heparin's extensively sulfated domains. The engineered cationic capsids retained their ability to inhibit heparin at high concentrations and showed no anticlotting activity of the kind that limits the utility of antiheparin polycationic agents that are currently in clinical use.
Figures
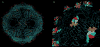


Similar articles
-
Heparin Binding to an Engineered Virus-like Nanoparticle Antagonist.Biomacromolecules. 2017 Dec 11;18(12):4113-4120. doi: 10.1021/acs.biomac.7b01174. Epub 2017 Oct 18. Biomacromolecules. 2017. PMID: 28949131
-
Engineered virus-like nanoparticle heparin antagonists.Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:4118-20. doi: 10.1109/EMBC.2013.6610451. Annu Int Conf IEEE Eng Med Biol Soc. 2013. PMID: 24110638
-
Directed polyvalent display of sulfated ligands on virus nanoparticles elicits heparin-like anticoagulant activity.Bioconjug Chem. 2014 Aug 20;25(8):1444-52. doi: 10.1021/bc500200t. Epub 2014 Jul 10. Bioconjug Chem. 2014. PMID: 24960223
-
Heparin: An old drug for new clinical applications.Carbohydr Polym. 2022 Nov 1;295:119818. doi: 10.1016/j.carbpol.2022.119818. Epub 2022 Jul 3. Carbohydr Polym. 2022. PMID: 35989029 Review.
-
111In-Labeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetracetic acid-Glu{PEG4-Glu[cyclo(Lys-Arg-Gly-Asp-d-Phe)]-cyclo(Lys-Arg-Gly-Asp-d-Phe)}-{PEG4-Glu[cyclo(Lys-Arg-Gly-Asp-d-Phe)]-cyclo(Lys-Arg-Gly-Asp-d-Phe)} (PEG4 = 15 amino-4,710,13-tetraoxapentadecanoic acid).2012 Feb 23 [updated 2012 Mar 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Feb 23 [updated 2012 Mar 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22457887 Free Books & Documents. Review.
Cited by
-
HEMA-Lysine-Based Cryogels for Highly Selective Heparin Neutralization.Int J Mol Sci. 2024 Jun 13;25(12):6503. doi: 10.3390/ijms25126503. Int J Mol Sci. 2024. PMID: 38928208 Free PMC article.
-
Evolution and protein packaging of small-molecule RNA aptamers.ACS Nano. 2011 Oct 25;5(10):7722-9. doi: 10.1021/nn2006927. Epub 2011 Sep 7. ACS Nano. 2011. PMID: 21899290 Free PMC article.
-
Biomolecular Assemblies: Moving from Observation to Predictive Design.Chem Rev. 2018 Dec 26;118(24):11519-11574. doi: 10.1021/acs.chemrev.8b00038. Epub 2018 Oct 3. Chem Rev. 2018. PMID: 30281290 Free PMC article. Review.
-
Cell targeting with hybrid Qβ virus-like particles displaying epidermal growth factor.Chembiochem. 2011 Nov 4;12(16):2441-7. doi: 10.1002/cbic.201100469. Epub 2011 Sep 29. Chembiochem. 2011. PMID: 21956837 Free PMC article.
-
Chemically orthogonal three-patch microparticles.Angew Chem Int Ed Engl. 2014 Feb 24;53(9):2332-8. doi: 10.1002/anie.201310727. Epub 2014 Feb 14. Angew Chem Int Ed Engl. 2014. PMID: 24574030 Free PMC article.
References
-
- Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. ACS Chem Biol. 2007;2:119–127. - PubMed
-
- Kawamura KS, Sung M, Bolewska-Pedyczak E, Gariepy J. Biochemistry. 2006;45:1116–1127. - PubMed
-
- Petty NK, Evans TJ, Fineran PC, Salmond GPC. Trends Biotechnol. 2006;24:7–15. - PubMed
-
- Pattenden LK, Middelberg APJ, Niebert M, Lipin DI. Trends Biotechnol. 2005;23:523–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

