The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces
- PMID: 19157536
- PMCID: PMC3679919
- DOI: 10.1016/j.biomaterials.2008.12.053
The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces
Abstract
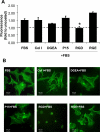
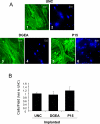
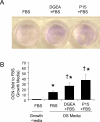
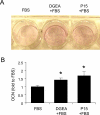
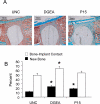
Integrin-binding peptides increase cell adhesion to naive hydroxyapatite (HA), however, in the body, HA becomes rapidly modified by protein adsorption. Previously we reported that, when combined with an adsorbed protein layer, RGD peptides interfered with cell adhesion to HA. In the current study we evaluated mesenchymal stem cell (MSC) interactions with HA disks coated with the collagen-mimetic peptides, DGEA, P15 and GFOGER. MSCs adhered equally well to disks coated with DGEA, P15, or collagen I, and all three substrates, but not GFOGER, supported greater cell adhesion than uncoated HA. When peptide-coated disks were overcoated with proteins from serum or the tibial microenvironment, collagen mimetics did not inhibit MSC adhesion, as was observed with RGD, however neither did they enhance adhesion. Given that activation of collagen-selective integrins stimulates osteoblastic differentiation, we monitored osteocalcin secretion and alkaline phosphatase activity from MSCs adherent to DGEA or P15-coated disks. Both of these osteoblastic markers were upregulated by DGEA and P15, in the presence and absence of differentiation-inducing media. Finally, bone formation on HA tibial implants was increased by the collagen mimetics. Collectively these results suggest that collagen-mimetic peptides improve osseointegration of HA, most probably by stimulating osteoblastic differentiation, rather than adhesion, of MSCs.
Figures



Similar articles
-
Enhancement of peptide coupling to hydroxyapatite and implant osseointegration through collagen mimetic peptide modified with a polyglutamate domain.Biomaterials. 2010 Dec;31(36):9586-94. doi: 10.1016/j.biomaterials.2010.08.020. Epub 2010 Oct 28. Biomaterials. 2010. PMID: 21035181 Free PMC article.
-
The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials.Biomaterials. 2008 Jul;29(21):3075-83. doi: 10.1016/j.biomaterials.2008.04.014. Epub 2008 Apr 25. Biomaterials. 2008. PMID: 18440064 Free PMC article.
-
Binding Peptide-Promoted Biofunctionalization of Graphene Paper with Hydroxyapatite for Stimulating Osteogenic Differentiation of Mesenchymal Stem Cells.ACS Appl Mater Interfaces. 2022 Jan 12;14(1):350-360. doi: 10.1021/acsami.1c20740. Epub 2021 Dec 28. ACS Appl Mater Interfaces. 2022. PMID: 34962367
-
Mesenchymal stem cell responses to bone-mimetic electrospun matrices composed of polycaprolactone, collagen I and nanoparticulate hydroxyapatite.PLoS One. 2011 Feb 8;6(2):e16813. doi: 10.1371/journal.pone.0016813. PLoS One. 2011. PMID: 21346817 Free PMC article.
-
The effect of the addition of a polyglutamate motif to RGD on peptide tethering to hydroxyapatite and the promotion of mesenchymal stem cell adhesion.Biomaterials. 2005 Dec;26(34):7046-56. doi: 10.1016/j.biomaterials.2005.05.006. Biomaterials. 2005. PMID: 15964067
Cited by
-
Osteogenic Peptides and Attachment Methods Determine Tissue Regeneration in Modified Bone Graft Substitutes.J Funct Biomater. 2021 Mar 31;12(2):22. doi: 10.3390/jfb12020022. J Funct Biomater. 2021. PMID: 33807267 Free PMC article. Review.
-
Carbon Nanostructures in Bone Tissue Engineering.Open Orthop J. 2016 Dec 30;10:877-899. doi: 10.2174/1874325001610010877. eCollection 2016. Open Orthop J. 2016. PMID: 28217212 Free PMC article.
-
Towards Stem Cell Therapy for Critical-Sized Segmental Bone Defects: Current Trends and Challenges on the Path to Clinical Translation.J Funct Biomater. 2024 May 27;15(6):145. doi: 10.3390/jfb15060145. J Funct Biomater. 2024. PMID: 38921519 Free PMC article. Review.
-
Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair.Biomaterials. 2010 Mar;31(9):2574-82. doi: 10.1016/j.biomaterials.2009.12.008. Epub 2009 Dec 28. Biomaterials. 2010. PMID: 20056517 Free PMC article.
-
Role of cell-matrix interactions on VIC phenotype and tissue deposition in 3D PEG hydrogels.J Tissue Eng Regen Med. 2016 Oct;10(10):E443-E453. doi: 10.1002/term.1836. Epub 2013 Oct 16. J Tissue Eng Regen Med. 2016. PMID: 24130082 Free PMC article.
References
-
- Sawyer AA, Hennessy KM, Bellis SL. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials. 2007;28(3):383–92. - PubMed
-
- Dalton BA, McFarland CD, Underwood PA, Steele JG. Role of the heparin binding domain of fibronectin in attachment and spreading of human bone-derived cells. J Cell Sci. 1995;108(Pt 5):2083–92. - PubMed
-
- Aota S, Nagai T, Yamada KM. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991;266(24):15938–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

