BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration
- PMID: 19158083
- PMCID: PMC2666605
- DOI: 10.1074/jbc.M809393200
BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration
Abstract
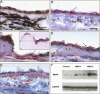
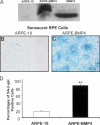
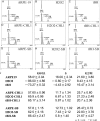
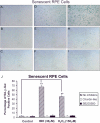
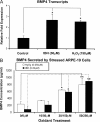
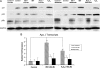
The retinal pigment epithelium is a primary site of pathology in age-related macular degeneration. Oxidative stress and senescence are both thought to be important mediators of macular degeneration pathogenesis. We demonstrate here that bone morphogenetic protein-4 is highly expressed in the retinal pigment epithelium and adjacent extracellular matrix of patients with dry age-related macular degeneration. In vitro studies revealed that sublethal oxidative stress increased bone morphogenetic protein-4 expression in retinal pigment epithelial cells, and both bone morphogenetic protein-4 and persistent mild oxidative stress can induce retinal pigment epithelial cell senescence through p53-p21(Cip1/WAF1)-Rb pathway. We further demonstrate that bone morphogenetic protein-4 acts as a mediator in oxidative stress-induced senescence and that this mediator function is via Smad and the p38 signaling pathway to increase and activate p53 and p21(Cip1/WAF1) and decrease phospho-Rb. Oxidative stress-induced senescence can be blocked by Chordin-like, an antagonist of bone morphogenetic protein-4, or SB203580, a phospho-p38 inhibitor. Our results suggest that oxidative stress and bone morphogenetic protein-4 may interact to promote retinal pigment epithelial cell senescence and that bone morphogenetic protein-4 may represent a novel therapeutic target to inhibit the progressive effects of oxidative stress and senescence in dry age-related macular degeneration.
Figures



References
-
- de Jong, P. T. (2006) N. Engl. J. Med. 355 1474-1485 - PubMed
-
- Klein, R., Klein, B. E., Jensen, S. C., and Meuer, S. M. (1997) Ophthalmology 104 7-21 - PubMed
-
- Ambati, J., Ambati, B. K., Yoo, S. H., Ianchulev, S., and Adamis, A. P. (2003) Surv. Ophthalmol. 48 257-293 - PubMed
-
- Zarbin, M. A. (2004) Arch. Ophthalmol. 122 598-614 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

