Coagulation factors IX and X enhance binding and infection of adenovirus types 5 and 31 in human epithelial cells
- PMID: 19158249
- PMCID: PMC2663266
- DOI: 10.1128/JVI.02562-08
Coagulation factors IX and X enhance binding and infection of adenovirus types 5 and 31 in human epithelial cells
Abstract
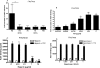
Most adenoviruses bind directly to the coxsackie and adenovirus receptor (CAR) on target cells in vitro, but recent research has shown that adenoviruses can also use soluble components in body fluids for indirect binding to target cells. These mechanisms have been identified upon addressing the questions of how to de- and retarget adenovirus-based vectors for human gene and cancer therapy, but the newly identified mechanisms also suggest that the role of body fluids and their components may also be of importance for natural, primary infections. Here we demonstrate that plasma, saliva, and tear fluid promote binding and infection of adenovirus type 5 (Ad5) in respiratory and ocular epithelial cells, which corresponds to the natural tropism of most adenoviruses, and that plasma promotes infection by Ad31. By using a set of binding and infection experiments, we also found that Ad5 and Ad31 require coagulation factors IX (FIX) or X (FX) or just FIX, respectively, for efficient binding and infection. The concentrations of these factors that were required for maximum binding were 1/100th of the physiological concentrations. Preincubation of virions with heparin or pretreatment of cells with heparinase I indicated that the role of cell surface heparan sulfate during FIX- and FX-mediated adenovirus binding and infection is mechanistically serotype specific. We conclude that the use of coagulation factors by adenoviruses may be of importance not only for the liver tropism seen when administering adenovirus vectors to the circulation but also during primary infections by wild-type viruses of their natural target cell types.
Figures
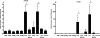
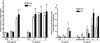


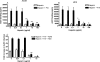

Similar articles
-
Requirements for receptor engagement during infection by adenovirus complexed with blood coagulation factor X.PLoS Pathog. 2010 Oct 7;6(10):e1001142. doi: 10.1371/journal.ppat.1001142. PLoS Pathog. 2010. PMID: 20949078 Free PMC article.
-
Coagulation factor IX mediates serotype-specific binding of species A adenoviruses to host cells.J Virol. 2011 Dec;85(24):13420-31. doi: 10.1128/JVI.06088-11. Epub 2011 Oct 5. J Virol. 2011. PMID: 21976659 Free PMC article.
-
Mouse adenovirus type 1 and human adenovirus type 5 differ in endothelial cell tropism and liver targeting.J Gene Med. 2009 Feb;11(2):119-27. doi: 10.1002/jgm.1283. J Gene Med. 2009. PMID: 19065608
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
-
The importance of coagulation factors binding to adenovirus: historical perspectives and implications for gene delivery.Expert Opin Drug Deliv. 2014 Nov;11(11):1795-813. doi: 10.1517/17425247.2014.938637. Epub 2014 Jul 18. Expert Opin Drug Deliv. 2014. PMID: 25036189 Review.
Cited by
-
Viral Infection and Antiviral Treatments in Ocular Pathologies.Microorganisms. 2022 Nov 10;10(11):2224. doi: 10.3390/microorganisms10112224. Microorganisms. 2022. PMID: 36363815 Free PMC article. Review.
-
Advances of Recombinant Adenoviral Vectors in Preclinical and Clinical Applications.Viruses. 2024 Feb 28;16(3):377. doi: 10.3390/v16030377. Viruses. 2024. PMID: 38543743 Free PMC article. Review.
-
Influence of Heparan Sulfate Proteoglycans and Factor X on species D Human Adenovirus Uptake and Transduction.Viruses. 2022 Dec 24;15(1):55. doi: 10.3390/v15010055. Viruses. 2022. PMID: 36680095 Free PMC article.
-
Proposed mechanism for rare thrombotic events after use of some Covid-19 vaccines.Med Hypotheses. 2022 Feb;159:110756. doi: 10.1016/j.mehy.2021.110756. Epub 2022 Jan 3. Med Hypotheses. 2022. PMID: 35002021 Free PMC article.
-
Interactions of viruses and the humoral innate immune response.Clin Immunol. 2020 Mar;212:108351. doi: 10.1016/j.clim.2020.108351. Epub 2020 Feb 4. Clin Immunol. 2020. PMID: 32028020 Free PMC article. Review.
References
-
- Araki-Sasaki, K., K. Y. Ohasi, T. Sasabe, K. Hayashi, H. Watanabe, Y. Tano, and H. Handa. 1995. An SV-40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. 36614-621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

