Dual roles for an arginine-rich motif in specific genome recognition and localization of viral coat protein to RNA replication sites in flock house virus-infected cells
- PMID: 19158251
- PMCID: PMC2655541
- DOI: 10.1128/JVI.01780-08
Dual roles for an arginine-rich motif in specific genome recognition and localization of viral coat protein to RNA replication sites in flock house virus-infected cells
Abstract
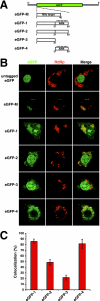
Assembly of many RNA viruses entails the encapsidation of multiple genome segments into a single virion, and underlying mechanisms for this process are still poorly understood. In the case of the nodavirus Flock House virus (FHV), a bipartite positive-strand RNA genome consisting of RNA1 and RNA2 is copackaged into progeny virions. In this study, we investigated whether the specific packaging of FHV RNA is dependent on an arginine-rich motif (ARM) located in the N terminus of the coat protein. Our results demonstrate that the replacement of all arginine residues within this motif with alanines rendered the resultant coat protein unable to package RNA1, suggesting that the ARM represents an important determinant for the encapsidation of this genome segment. In contrast, replacement of all arginines with lysines had no effect on RNA1 packaging. Interestingly, confocal microscopic analysis demonstrated that the RNA1 packaging-deficient mutant did not localize to mitochondrial sites of FHV RNA replication as efficiently as wild-type coat protein. In addition, gain-of-function analyses showed that the ARM by itself was sufficient to target green fluorescent protein to RNA replication sites. These data suggest that the packaging of RNA1 is dependent on trafficking of coat protein to mitochondria, the presumed site of FHV assembly, and that this trafficking requires a high density of positive charge in the N terminus. Our results are compatible with a model in which recognition of RNA1 and RNA2 for encapsidation occurs sequentially and in distinct cellular microenvironments.
Figures






Similar articles
-
Assembly of two independent populations of flock house virus particles with distinct RNA packaging characteristics in the same cell.J Virol. 2007 Jan;81(2):613-9. doi: 10.1128/JVI.01668-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079301 Free PMC article.
-
Differential segregation of nodaviral coat protein and RNA into progeny virions during mixed infection with FHV and NoV.Virology. 2014 Apr;454-455:280-90. doi: 10.1016/j.virol.2014.03.003. Epub 2014 Mar 21. Virology. 2014. PMID: 24725955 Free PMC article.
-
Analysis of RNA packaging in wild-type and mosaic protein capsids of flock house virus using recombinant baculovirus vectors.Virology. 2003 Jan 5;305(1):10-24. doi: 10.1006/viro.2002.1740. Virology. 2003. PMID: 12504536
-
Recent insights into the biology and biomedical applications of Flock House virus.Cell Mol Life Sci. 2008 Sep;65(17):2675-87. doi: 10.1007/s00018-008-8037-y. Cell Mol Life Sci. 2008. PMID: 18516498 Free PMC article. Review.
-
Cryo-electron microscopy of nodavirus RNA replication organelles illuminates positive-strand RNA virus genome replication.Curr Opin Virol. 2021 Dec;51:74-79. doi: 10.1016/j.coviro.2021.09.008. Epub 2021 Sep 30. Curr Opin Virol. 2021. PMID: 34601307 Free PMC article. Review.
Cited by
-
Subcellular localization and rearrangement of endoplasmic reticulum by Brome mosaic virus capsid protein.J Virol. 2011 Mar;85(6):2953-63. doi: 10.1128/JVI.02020-10. Epub 2011 Jan 5. J Virol. 2011. PMID: 21209103 Free PMC article.
-
Packaging host RNAs in small RNA viruses: an inevitable consequence of an error-prone polymerase?Cell Cycle. 2012 Oct 15;11(20):3713-4. doi: 10.4161/cc.22112. Epub 2012 Sep 14. Cell Cycle. 2012. PMID: 22983002 Free PMC article. No abstract available.
-
Multifunctional Protein A Is the Only Viral Protein Required for Nodavirus RNA Replication Crown Formation.Viruses. 2022 Dec 3;14(12):2711. doi: 10.3390/v14122711. Viruses. 2022. PMID: 36560715 Free PMC article.
-
An examination of the electrostatic interactions between the N-terminal tail of the Brome Mosaic Virus coat protein and encapsidated RNAs.J Mol Biol. 2012 Jun 22;419(5):284-300. doi: 10.1016/j.jmb.2012.03.023. Epub 2012 Apr 1. J Mol Biol. 2012. PMID: 22472420 Free PMC article.
-
Rapid evolution of virus sequences in intrinsically disordered protein regions.PLoS Pathog. 2014 Dec 11;10(12):e1004529. doi: 10.1371/journal.ppat.1004529. eCollection 2014 Dec. PLoS Pathog. 2014. PMID: 25502394 Free PMC article.
References
-
- Ball, L. A., and K. L. Johnson. 1998. Nodaviruses of insects, p. 225-267. In L. K. Miller and L. A. Ball (ed.), The insect viruses. Plenum, New York, NY.
-
- Basnayake, V. R., T. L. Sit, and S. A. Lommel. 2006. The genomic RNA packaging scheme of Red clover necrotic mosaic virus. Virology 345532-539. - PubMed
-
- Battiste, J. L., H. Mao, N. S. Rao, R. Tan, D. R. Muhandiram, L. E. Kay, A. D. Frankel, and J. R. Williamson. 1996. Alpha helix-RNA major groove recognition in an HIV-1 Rev peptide-RRE RNA complex. Science 2731547-1551. - PubMed
-
- Cai, Z., A. Gorin, R. Frederick, X. Ye, W. Hu, A. Majumdar, A. Kettani, and D. J. Patel. 1998. Solution structure of P22 transcriptional antitermination N peptide-box B RNA complex. Nat. Struct. Biol. 5203-212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

