Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta
- PMID: 19158279
- PMCID: PMC2655609
- DOI: 10.1128/MCB.01320-08
Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta
Abstract
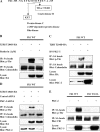
Previous studies have shown that transforming growth factor beta (TGF-beta)-induced collagen gene expression involves acetylation-dependent dissociation from the human alpha2(I) collagen (COL1A2) promoter of the transcriptional repressor Fli1. The goal of this study was to elucidate the regulatory steps preceding the acetylation of Fli1. We first showed that TGF-beta induces Fli1 phosphorylation on a threonine residue(s). The major phosphorylation site was localized to threonine 312 located in the DNA binding domain of Fli1. Using several independent approaches, we demonstrated that Fli1 is directly phosphorylated by protein kinase C delta (PKC delta). Additional experiments showed that in response to TGF-beta, PKC delta is recruited to the collagen promoter to phosphorylate Fli1 and that this step is a prerequisite for the subsequent interaction of Fli1 with p300/CREB-binding protein-associated factor (PCAF) and an acetylation event. The phosphorylation of endogenous Fli1 preceded its acetylation in response to TGF-beta stimulation, and the blockade of PKC delta abrogated both the phosphorylation and acetylation of Fli1 in dermal fibroblasts. Promoter studies showed that a phosphorylation-deficient mutant of Fli1 exhibited an increased inhibitory effect on the COL1A2 gene, which could not be reversed by the forced expression of PCAF or PKC delta. These data strongly suggest that the phosphorylation-acetylation cascade triggered by PKC delta represents the primary mechanism whereby TGF-beta regulates the transcriptional activity of Fli1 in the context of the collagen promoter.
Figures




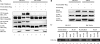




References
-
- Asano, Y., J. Czuwara, and M. Trojanowska. 2007. Transforming growth factor-beta regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J. Biol. Chem. 28234672-34683. - PubMed
-
- Asano, Y., H. Ihn, K. Yamane, M. Jinnin, Y. Mimura, and K. Tamaki. 2004. Phosphatidylinositol 3-kinase is involved in alpha2(I) collagen gene expression in normal and scleroderma fibroblasts. J. Immunol. 1727123-7135. - PubMed
-
- Bailly, R.-A., R. Bosselut, J. Zucman, F. Cormier, O. Delattre, M. Roussel, G. Thomas, and J. Ghysdael. 1994. DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Mol. Cell. Biol. 143230-3241. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
