HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine
- PMID: 19158287
- PMCID: PMC2744993
- DOI: 10.1523/JNEUROSCI.3481-08.2009
HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine
Abstract
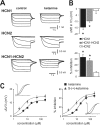
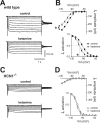
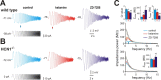
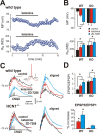
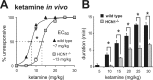
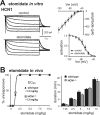
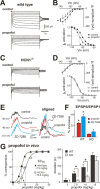
Ketamine has important anesthetic, analgesic, and psychotropic actions. It is widely believed that NMDA receptor inhibition accounts for ketamine actions, but there remains a dearth of behavioral evidence to support this hypothesis. Here, we present an alternative, behaviorally relevant molecular substrate for anesthetic effects of ketamine: the HCN1 pacemaker channels that underlie a neuronal hyperpolarization-activated cationic current (I(h)). Ketamine caused subunit-specific inhibition of recombinant HCN1-containing channels and neuronal I(h) at clinically relevant concentrations; the channels were more potently inhibited by S-(+)-ketamine than racemic ketamine, consistent with anesthetic actions of the compounds. In cortical pyramidal neurons from wild-type, but not HCN1 knock-out mice, ketamine induced membrane hyperpolarization and enhanced dendritosomatic synaptic coupling; both effects are known to promote cortical synchronization and support slow cortical rhythms, like those accompanying anesthetic-induced hypnosis. Accordingly, we found that the potency for ketamine to provoke a loss-of-righting reflex, a behavioral correlate of hypnosis, was strongly reduced in HCN1 knock-out mice. In addition, hypnotic sensitivity to two other intravenous anesthetics in HCN1 knock-out mice matched effects on HCN1 channels; propofol selectively inhibited HCN1 channels and propofol sensitivity was diminished in HCN1 knock-out mice, whereas etomidate had no effect on HCN1 channels and hypnotic sensitivity to etomidate was unaffected by HCN1 gene deletion. These data advance HCN1 channels as a novel molecular target for ketamine, provide a plausible neuronal mechanism for enhanced cortical synchronization during anesthetic-induced hypnosis and suggest that HCN1 channels might contribute to other unexplained actions of ketamine.
Figures
References
-
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. - PubMed
-
- Antkowiak B. In vitro networks: cortical mechanisms of anaesthetic action. Br J Anaesth. 2002;89:102–111. - PubMed
-
- Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb Cortex. 2007;17:702–712. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
