Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo
- PMID: 19158294
- PMCID: PMC2719435
- DOI: 10.1523/JNEUROSCI.5060-08.2009
Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo
Abstract
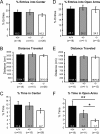
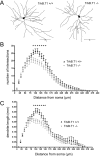
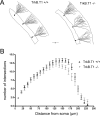
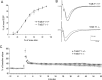
Pathological or in vitro overexpression of the truncated TrkB (TrkB.T1) receptor inhibits signaling through the full-length TrkB (TrkB.FL) tyrosine kinase receptor. However, to date, the role of endogenous TrkB.T1 is still unknown. By studying mice lacking the truncated TrkB.T1 isoform but retaining normal spatiotemporal expression of TrkB.FL, we have analyzed TrkB.T1-specific physiological functions and its effect on endogenous TrkB kinase signaling in vivo. We found that TrkB.T1-deficient mice develop normally but show increased anxiety in association with morphological abnormalities in the length and complexity of neurites of neurons in the basolateral amygdala. However, no behavioral abnormalities were detected in hippocampal-dependent memory tasks, which correlated with lack of any obvious hippocampal morphological deficits or alterations in basal synaptic transmission and long-term potentiation. In vivo reduction of TrkB signaling by removal of one BDNF allele could be partially rescued by TrkB.T1 deletion, which was revealed by an amelioration of the enhanced aggression and weight gain associated with BDNF haploinsufficiency. Our results suggest that, at the physiological level, TrkB.T1 receptors are important regulators of TrkB.FL signaling in vivo. Moreover, TrkB.T1 selectively affects dendrite complexity of certain neuronal populations.
Figures
References
-
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. - PubMed
-
- Biffo S, Offenhäuser N, Carter BD, Barde YA. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121:2461–2470. - PubMed
-
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
