SCAMP3 negatively regulates epidermal growth factor receptor degradation and promotes receptor recycling
- PMID: 19158374
- PMCID: PMC2655259
- DOI: 10.1091/mbc.e08-09-0894
SCAMP3 negatively regulates epidermal growth factor receptor degradation and promotes receptor recycling
Abstract
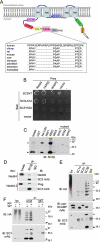
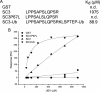
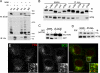
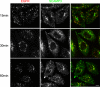
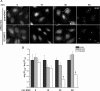
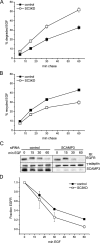
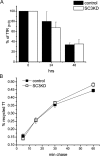
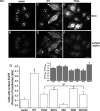
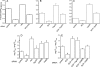
The epidermal growth factor receptor (EGFR) is targeted for lysosomal degradation by ubiquitin-mediated interactions with the ESCRTs (endosomal-sorting complexes required for transport) in multivesicular bodies (MVBs). We show that secretory carrier membrane protein, SCAMP3, localizes in part to early endosomes and negatively regulates EGFR degradation through processes that involve its ubiquitylation and interactions with ESCRTs. SCAMP3 is multimonoubiquitylated and is able to associate with Nedd4 HECT ubiquitin ligases and the ESCRT-I subunit Tsg101 via its PY and PSAP motifs, respectively. SCAMP3 also associates with the ESCRT-0 subunit Hrs. Depletion of SCAMP3 in HeLa cells by inhibitory RNA accelerated degradation of EGFR and EGF while inhibiting recycling. Conversely, overexpression enhanced EGFR recycling unless ubiquitylatable lysines, PY or PSAP motifs in SCAMP3 were mutated. Notably, dual depletions of SCAMP3 and ESCRT subunits suggest that SCAMP3 has a distinct function in parallel with the ESCRTs that regulates receptor degradation. This function may affect trafficking of receptors from prelysosomal compartments as SCAMP3 depletion appeared to sustain the incidence of EGFR-containing MVBs detected by immunoelectron microscopy. Together, our results suggest that SCAMP3, its modification with ubiquitin, and its interactions with ESCRTs coordinately regulate endosomal pathways and affect the efficiency of receptor down-regulation.
Figures

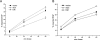


References
-
- Alwan H. A., van Leeuwen J. E. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J. Biol. Chem. 2007;282:1658–1669. - PubMed
-
- Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. - PubMed
-
- Bache K. G., Raiborg C., Mehlum A., Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 2003b;278:12513–12521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

