Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells
- PMID: 19158378
- PMCID: PMC2655261
- DOI: 10.1091/mbc.e08-10-1048
Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells
Abstract
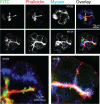
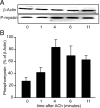
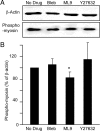
Many studies have implicated F-actin and myosin 2 in the control of regulated secretion. Most recently, evidence suggests a role for the microfilament network in regulating the postfusion events of vesicle dynamics. This is of potential importance as postfusion behavior can influence the loss of vesicle content and may provide a new target for drug therapy. We have investigated the role of myosin 2 in regulating exocytosis in secretory epithelial cells by using novel assays to determine the behavior of the fusion pore in individual granules. We immunolocalize myosin 2A to the apical region of pancreatic acinar cells, suggesting it is this isoform that plays a role in granule exocytosis. We further show myosin 2 phosphorylation increased on cell stimulation, consistent with a regulatory role in secretion. Importantly, in a single-cell, single-granule secretion assay, neither the myosin 2 inhibitor (-)-blebbistatin nor the myosin light chain kinase inhibitor ML-9 had any effect on the numbers of granules stimulated to fuse after cell stimulation. These data indicate that myosin 2, if it has any action on secretion, must be targeting postfusion granule behavior. This interpretation is supported by direct study of fusion pore opening in which we show that (-)-blebbistatin and ML-9 promote fusion pore closure and decrease fusion pore lifetimes. Our work now adds to a growing body of evidence showing that myosin 2 is an essential regulator of postfusion granule behavior. In particular, in the case of the secretory epithelial cells, myosin 2 activity is necessary to maintain fusion pore opening.
Figures
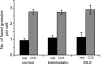
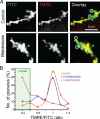
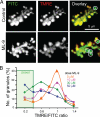

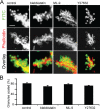
References
-
- Albillos A., Dernick G., Horstmann H., Almers W., Alvarez de Toldeo G., Lindau M. The exocytic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
-
- Alés E., Tabares L., Poyato J. M., Valero V., Lindau M., Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. - PubMed
-
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. - PubMed
-
- Archer D. A., Graham M. E., Burgoyne R. D. Complexin regulates the closure of the fusion pore during regulated vesicle exocytosis. J. Biol. Chem. 2002;277:18249–18252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

