Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis
- PMID: 19158379
- PMCID: PMC2655252
- DOI: 10.1091/mbc.e08-10-1033
Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis
Abstract
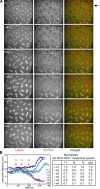
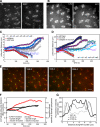
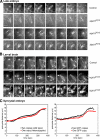
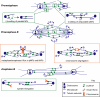
We used antibody microinjection and genetic manipulations to dissect the various roles of the homotetrameric kinesin-5, KLP61F, in astral, centrosome-controlled Drosophila embryo spindles and to test the hypothesis that it slides apart interpolar (ip) microtubules (MT), thereby controlling poleward flux and spindle length. In wild-type and Ncd null mutant embryos, anti-KLP61F dissociated the motor from spindles, producing a spatial gradient in the KLP61F content of different spindles, which was visible in KLP61F-GFP transgenic embryos. The resulting mitotic defects, supported by gene dosage experiments and time-lapse microscopy of living klp61f mutants, reveal that, after NEB, KLP61F drives persistent MT bundling and the outward sliding of antiparallel MTs, thereby contributing to several processes that all appear insensitive to cortical disruption. KLP61F activity contributes to the poleward flux of both ipMTs and kinetochore MTs and to the length of the metaphase spindle. KLP61F activity maintains the prometaphase spindle by antagonizing Ncd and another unknown force-generator and drives anaphase B, although the rate of spindle elongation is relatively insensitive to the motor's concentration. Finally, KLP61F activity contributes to normal chromosome congression, kinetochore spacing, and anaphase A rates. Thus, a KLP61F-driven sliding filament mechanism contributes to multiple aspects of mitosis in this system.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

