The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles
- PMID: 19158398
- PMCID: PMC2655250
- DOI: 10.1091/mbc.e08-03-0282
The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles
Abstract
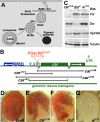
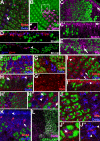
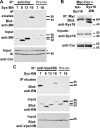
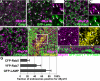
The SM proteins Vps33A and Vps33B are believed to act in membrane fusions in endosomal pathways, but their specific roles are controversial. In Drosophila, Vps33A is the product of the carnation (car) gene. We generated a null allele of car to test its requirement for trafficking to different organelles. Complete loss of car function is lethal during larval development. Eye-specific loss of Car causes late, light-independent degeneration of photoreceptor cells. Earlier in these cells, two distinct phenotypes were detected. In young adults, autophagosomes amassed indicating that their fusion with lysosomes requires Car. In eye discs, endocytosed receptors and ligands accumulate in Rab7-positive prelysosomal compartments. The requirement of Car for late endosome-to-lysosome fusion in imaginal discs is specific as early endosomes are unaffected. Furthermore, lysosomal delivery is not restored by expression of dVps33B. This specificity reflects the distinct pattern of binding to different Syntaxins in vitro: dVps33B predominantly binds the early endosomal Avl and Car to dSyntaxin16. Consistent with a role in Car-mediated fusion, dSyntaxin16 is not restricted to Golgi membranes but also present on lysosomes.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

