Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance
- PMID: 19158483
- PMCID: PMC3041590
- DOI: 10.4161/cc.8.1.7533
Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance
Abstract
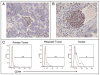
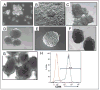
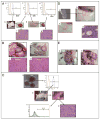
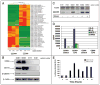
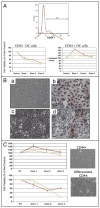
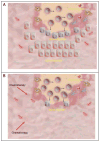
A major burden in the treatment of ovarian cancer is the high percentage of recurrence and chemoresistance. Cancer stem cells (CSCs) provide a reservoir of cells that can self-renew, can maintain the tumor by generating differentiated cells [non-stem cells (non-CSCs)] which make up the bulk of the tumor and may be the primary source of recurrence. We describe the characterization of human ovarian cancer stem cells (OCSCs). These cells have a distinctive genetic profile that confers them with the capacity to recapitulate the original tumor, proliferate with chemotherapy, and promote recurrence. CSC identified in EOC cells isolated form ascites and solid tumors are characterized by: CD44+, MyD88+, constitutive NFkappaB activity and cytokine and chemokine production, high capacity for repair, chemoresistance to conventional chemotherapies, resistance to TNFalpha-mediated apoptosis, capacity to form spheroids in suspension, and the ability to recapitulate in vivo the original tumor. Chemotherapy eliminates the bulk of the tumor but it leaves a core of cancer cells with high capacity for repair and renewal. The molecular properties identified in these cells may explain some of the unique characteristics of CSCs that control self-renewal and drive metastasis. The identification and cloning of human OCSCs can aid in the development of better therapeutic approaches for ovarian cancer patients.
Figures
References
-
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. - PubMed
-
- Onnis A. The management of ovarian cancer: an update. Eur J Gynaecol Oncol. 1997;18:157–60. - PubMed
-
- Torri V, Harper PG, Colombo N, Sandercock J, Parmar MK. Paclitaxel and cisplatin in ovarian cancer. J Clin Oncol. 2000;18:2349–51. - PubMed
-
- Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. Combination chemotherapy with carboplatin and docetaxel in the treatment of cancers of the ovary and fallopian tube and primary carcinoma of the peritoneum. J Clin Oncol. 2001;19:1901–5. - PubMed
-
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
