Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity
- PMID: 19158795
- PMCID: PMC2705195
- DOI: 10.1038/nature07664
Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity
Abstract
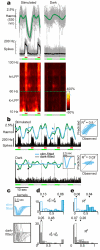
Haemodynamic signals underlying functional brain imaging (for example, functional magnetic resonance imaging (fMRI)) are assumed to reflect metabolic demand generated by local neuronal activity, with equal increases in haemodynamic signal implying equal increases in the underlying neuronal activity. Few studies have compared neuronal and haemodynamic signals in alert animals to test for this assumed correspondence. Here we present evidence that brings this assumption into question. Using a dual-wavelength optical imaging technique that independently measures cerebral blood volume and oxygenation, continuously, in alert behaving monkeys, we find two distinct components to the haemodynamic signal in the alert animals' primary visual cortex (V1). One component is reliably predictable from neuronal responses generated by visual input. The other component-of almost comparable strength-is a hitherto unknown signal that entrains to task structure independently of visual input or of standard neural predictors of haemodynamics. This latter component shows predictive timing, with increases of cerebral blood volume in anticipation of trial onsets even in darkness. This trial-locked haemodynamic signal could be due to an accompanying V1 arterial pumping mechanism, closely matched in time, with peaks of arterial dilation entrained to predicted trial onsets. These findings (tested in two animals) challenge the current understanding of the link between brain haemodynamics and local neuronal activity. They also suggest the existence of a novel preparatory mechanism in the brain that brings additional arterial blood to cortex in anticipation of expected tasks.
Figures



Comment in
-
Neuroscience: Pre-emptive blood flow.Nature. 2009 Jan 22;457(7228):387-8. doi: 10.1038/457387a. Nature. 2009. PMID: 19158777 Free PMC article.
References
-
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–769. - PubMed
-
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. - PubMed
-
- Vanzetta I, Grinvald A. Increased cortical oxidative metabolism due to sensory stimulation: implications for functional brain imaging. Science. 1999;286:1555–1558. - PubMed
-
- Ugurbil K, Toth LJ, Kim D-S. How accurate is magnetic resonance imaging of brain function. Trends Neurosi. 2003;26(2):108–114. - PubMed
-
- Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis for brain activity: implications for neuroimaging. Trends Neurosi. 2004;27(8):489–495. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

