Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia
- PMID: 19158945
- PMCID: PMC2617762
- DOI: 10.1371/journal.pone.0004253
Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia
Abstract
Background: Friedreich ataxia originates from a decrease in mitochondrial frataxin, which causes the death of a subset of neurons. The biochemical hallmarks of the disease include low activity of the iron sulfur cluster-containing proteins (ISP) and impairment of antioxidant defense mechanisms that may play a major role in disease progression.
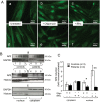
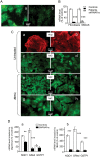
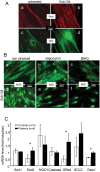
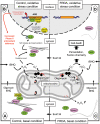
Methodology/principal findings: We thus investigated signaling pathways involved in antioxidant defense mechanisms. We showed that cultured fibroblasts from patients with Friedreich ataxia exhibited hypersensitivity to oxidative insults because of an impairment in the Nrf2 signaling pathway, which led to faulty induction of antioxidant enzymes. This impairment originated from previously reported actin remodeling by hydrogen peroxide.
Conclusions/significance: Thus, the defective machinery for ISP synthesis by causing mitochondrial iron dysmetabolism increases hydrogen peroxide production that accounts for the increased susceptibility to oxidative stress.
Conflict of interest statement
Figures

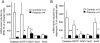
References
-
- Tzagoloff A. Mitochondria. New York: Plenum Press; 1982.
-
- Rustin P. Mitochondria, from cell death to proliferation. Nat Genet. 2002;30:352–3. - PubMed
-
- Koehler CM, Beverly KN, Leverich EP. Redox pathways of the mitochondrion. Antioxid Redox Signal. 2006;8:813–22. - PubMed
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

