Campylobacter jejuni PglH is a single active site processive polymerase that utilizes product inhibition to limit sequential glycosyl transfer reactions
- PMID: 19159314
- PMCID: PMC2736683
- DOI: 10.1021/bi802284d
Campylobacter jejuni PglH is a single active site processive polymerase that utilizes product inhibition to limit sequential glycosyl transfer reactions
Abstract
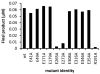
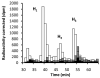
Asparagine-linked protein glycosylation is essential for the virulence of the human gut mucosal pathogen Campylobacter jejuni . The heptasaccharide that is transferred to proteins is biosynthesized via the glycosyltransferase-catalyzed addition of sugar units to an undecaprenyl diphosphate-linked carrier. Genetic studies on the heptasaccharide assembly enzymes have shown that PglH, which transfers three terminal N-acetyl-galactosamine (GalNAc) residues to the carrier polyisoprene, is essential for chick colonization by C. jejuni . While it is now clear that PglH catalyzes multiple transfer reactions, the mechanism whereby the reactions cease after the addition of just three GalNAc residues has yet to be understood. To address this issue, a series of mechanistic biochemical studies was conducted with purified native PglH. This enzyme was found to follow a processive mechanism under initial rate conditions; however, product inhibition and product accumulation led to PglH release of intermediate products prior to complete conversion to the native ultimate product. Point mutations of an essential EX(7)E sequence motif were used to demonstrate that a single active site was responsible for all three transferase reactions, and a homology model with the mannosyltransferase PimA, from Mycobacteria smegmatis , establishes the requirement of the EX(7)E motif in catalysis. Finally, increased binding affinity with increasing glycan size is proposed to provide PglH with a counting mechanism that does not allow the transfer of more than three GalNAc residues. These results provide important mechanistic insights into the function of the glycosyl transfer polymerase that is related to the virulence of C. jejuni .
Figures


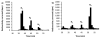
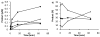
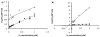
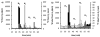
Similar articles
-
Structural basis of the molecular ruler mechanism of a bacterial glycosyltransferase.Nat Commun. 2018 Jan 31;9(1):445. doi: 10.1038/s41467-018-02880-2. Nat Commun. 2018. PMID: 29386647 Free PMC article.
-
Direct biochemical evidence for the utilization of UDP-bacillosamine by PglC, an essential glycosyl-1-phosphate transferase in the Campylobacter jejuni N-linked glycosylation pathway.Biochemistry. 2006 Apr 25;45(16):5343-50. doi: 10.1021/bi0602056. Biochemistry. 2006. PMID: 16618123
-
In vitro assembly of the undecaprenylpyrophosphate-linked heptasaccharide for prokaryotic N-linked glycosylation.Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14255-9. doi: 10.1073/pnas.0507311102. Epub 2005 Sep 26. Proc Natl Acad Sci U S A. 2005. PMID: 16186480 Free PMC article.
-
Dual Glycosyltransferases from Campylobacter concisus Diverge from the Canonical Campylobacter N-Linked Glycan Assembly Pathway.Biochemistry. 2024 Sep 17;63(18):2369-2379. doi: 10.1021/acs.biochem.4c00351. Epub 2024 Aug 28. Biochemistry. 2024. PMID: 39192839
-
Campylobacter jejuni: targeting host cells, adhesion, invasion, and survival.Appl Microbiol Biotechnol. 2023 May;107(9):2725-2754. doi: 10.1007/s00253-023-12456-w. Epub 2023 Mar 21. Appl Microbiol Biotechnol. 2023. PMID: 36941439 Free PMC article. Review.
Cited by
-
Composition of the Holdfast Polysaccharide from Caulobacter crescentus.J Bacteriol. 2019 Aug 8;201(17):e00276-19. doi: 10.1128/JB.00276-19. Print 2019 Sep 1. J Bacteriol. 2019. PMID: 31209074 Free PMC article.
-
Structural and mechanistic studies of the N-glycosylation machinery: from lipid-linked oligosaccharide biosynthesis to glycan transfer.Glycobiology. 2023 Dec 25;33(11):861-872. doi: 10.1093/glycob/cwad053. Glycobiology. 2023. PMID: 37399117 Free PMC article. Review.
-
Processivity in Bacterial Glycosyltransferases.ACS Chem Biol. 2020 Jan 17;15(1):3-16. doi: 10.1021/acschembio.9b00619. Epub 2019 Dec 11. ACS Chem Biol. 2020. PMID: 31750644 Free PMC article. Review.
-
Monitoring processivity and length control of a carbohydrate polymerase.J Am Chem Soc. 2011 Aug 17;133(32):12758-66. doi: 10.1021/ja204448t. Epub 2011 Jul 25. J Am Chem Soc. 2011. PMID: 21739979 Free PMC article.
-
Structural basis of inhibition of lipid-linked oligosaccharide flippase PglK by a conformational nanobody.Sci Rep. 2017 Apr 19;7:46641. doi: 10.1038/srep46641. Sci Rep. 2017. PMID: 28422165 Free PMC article.
References
-
- Sun J, Duffy KE, Ranjith-Kumar CT, Xiong J, Lamb RJ, Santos J, Masarapu H, Cunningham M, Holzenburg A, Sarisky RT, Mbow ML, Kao C. Structural and functional analyses of the human Toll-like receptor 3. Role of glycosylation. J Biol Chem. 2006;281:11144–11151. - PubMed
-
- Clevestig P, Pramanik L, Leitner T, Ehrnst A. CCR5 use by human immunodeficiency virus type 1 is associated closely with the gp120 V3 loop N-linked glycosylation site. J Gen Virol. 2006;87:607–612. - PubMed
-
- Torres GE, Egan TM, Voigt MM. N-Linked glycosylation is essential for the functional expression of the recombinant P2X2 receptor. Biochemistry. 1998;37:14845–14851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

