Pressure-overload magnitude-dependence of the anti-hypertrophic efficacy of PDE5A inhibition
- PMID: 19159628
- PMCID: PMC2703675
- DOI: 10.1016/j.yjmcc.2008.12.008
Pressure-overload magnitude-dependence of the anti-hypertrophic efficacy of PDE5A inhibition
Abstract
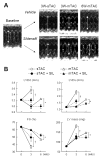
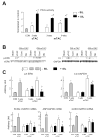
Increased myocardial cGMP, achieved by enhancing cyclase activity or impeding cGMP hydrolysis by phosphodiesterase type-5 (PDE5A), suppresses cellular and whole organ hypertrophy. The efficacy of the latter also requires cyclase stimulation and may depend upon co-activation of maladaptive signaling suppressible by cGMP-stimulated kinase (cGK-1). Thus, PDE5A inhibitors could paradoxically be more effective against higher than lower magnitudes of pressure-overload stress. To test this, mice were subjected to severe or moderate trans-aortic constriction (sTAC, mTAC) for 6 wks +/-co-treatment with oral sildenafil (SIL 200 mg/kg/d). LV mass (LVM) rose 130% after 3-wks sTAC and SIL blunted this by 50%. With mTAC, LVM rose 56% at 3 wks but was unaffected by SIL, whereas a 90% increase in LVM after 6 wks was suppressed by SIL. SIL minimally altered LV function and remodeling with mTAC until later stages that stimulated more hypertrophy and remodeling. SIL stimulated cGK-1 activity similarly at 3 and 6 wks of mTAC. However, pathologic stress signaling (e.g. calcineurin, ERK-MAPkinase) was little activated after 3-wk mTAC, unlike sTAC or later stage mTAC when activity increased and SIL suppressed it. With modest hypertrophy (3-wk mTAC), GSK3beta and Akt phosphorylation were unaltered but SIL enhanced it. However, with more severe hypertrophy (6-wk mTAC and 3-wk sTAC), both kinases were highly phosphorylated and SIL treatment reduced it. Thus, PDE5A-inhibition counters cardiac pressure-overload stress remodeling more effectively at higher than lower magnitude stress, coupled to pathologic signaling activation targetable by cGK-1 stimulation. Such regulation could impact responses of varying disease models to PDE5A inhibitors.
Figures


References
-
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. - PubMed
-
- Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. - PubMed
-
- McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nature Reviews Drug Discovery. 2007;6:1–18. - PubMed
-
- Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res. 2003;93:907–916. - PubMed
-
- Kass DA, Takimoto E, Nagayama T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75:303–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

