A genetically encoded calcium indicator for chronic in vivo two-photon imaging
- PMID: 19160515
- PMCID: PMC7618336
- DOI: 10.1038/nmeth.1243
A genetically encoded calcium indicator for chronic in vivo two-photon imaging
Abstract
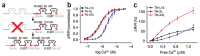
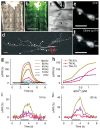
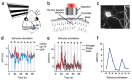
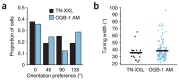
Neurons in the nervous system can change their functional properties over time. At present, there are no techniques that allow reliable monitoring of changes within identified neurons over repeated experimental sessions. We increased the signal strength of troponin C-based calcium biosensors in the low-calcium regime by mutagenesis and domain rearrangement within the troponin C calcium binding moiety to generate the indicator TN-XXL. Using in vivo two-photon ratiometric imaging, we show that TN-XXL exhibits enhanced fluorescence changes in neurons of flies and mice. TN-XXL could be used to obtain tuning curves of orientation-selective neurons in mouse visual cortex measured repeatedly over days and weeks. Thus, the genetically encoded calcium indicator TN-XXL allows repeated imaging of response properties from individual, identified neurons in vivo, which will be crucial for gaining new insights into cellular mechanisms of plasticity, regeneration and disease.
Conflict of interest statement
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at
Figures
References
-
- Kerr JND, Denk W. Imaging in vivo: watching the brain in action. Nat Rev Neurosci. 2008;9:195–205. - PubMed
-
- Ohki K, Chung S, Ch’Ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. - PubMed
-
- Ohki K, et al. Highly ordered arrangement of single neurons in orientation pinwheels. Nature. 2006;442:925–928. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

