Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects
- PMID: 19161395
- PMCID: PMC2818790
- DOI: 10.1111/j.1751-1097.2008.00507.x
Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects
Abstract
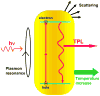
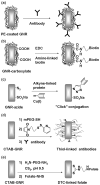
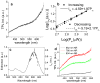
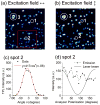
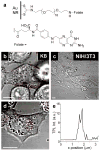
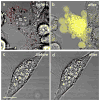
Gold nanorods (NRs) have plasmon-resonant absorption and scattering in the near-infrared (NIR) region, making them attractive probes for in vitro and in vivo imaging. In the cellular environment, NRs can provide scattering contrast for darkfield microscopy, or emit a strong two-photon luminescence due to plasmon-enhanced two-photon absorption. NRs have also been employed in biomedical imaging modalities such as optical coherence tomography or photoacoustic tomography. Careful control over surface chemistry enhances the capacity of NRs as biological imaging agents by enabling cell-specific targeting, and by increasing their dispersion stability and circulation lifetimes. NRs can also efficiently convert optical energy into heat, and inflict localized damage to tumor cells. Laser-induced heating of NRs can disrupt cell membrane integrity and homeostasis, resulting in Ca(2+) influx and the depolymerization of the intracellular actin network. The combination of plasmon-resonant optical properties, intense local photothermal effects and robust surface chemistry render gold NRs as promising theragnostic agents.
Figures




References
-
- Murphy CJ, Sau TK, Gole AM, Orendorff CJ, Gao J, Gou L, Hunyadi SE, Li T. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B. 2005;109:13857–13870. - PubMed
-
- Pérez-Juste J, Pastoriza-Santos I, Liz-Marzán LM, Mulvaney P. Gold nanorods: synthesis, characterization and applications. Coord Chem Rev. 2005;249:1870–1901.
-
- Hirsch LR, Gobin AM, Lowery AR, Tam F, Drezek RA, Halas NJ, West JL. Metal nanoshells. Annals Biomed Eng. 2006;34:15–22. - PubMed
-
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomed. 2007;2:681–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

