Cerebral microbleeds: a guide to detection and interpretation
- PMID: 19161908
- PMCID: PMC3414436
- DOI: 10.1016/S1474-4422(09)70013-4
Cerebral microbleeds: a guide to detection and interpretation
Abstract
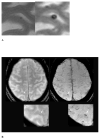
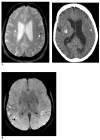
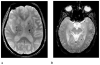
Cerebral microbleeds (CMBs) are increasingly recognised neuroimaging findings in individuals with cerebrovascular disease and dementia, and in normal ageing. There has been substantial progress in the understanding of CMBs in recent years, particularly in the development of newer MRI methods for the detection of CMBs and the application of these techniques to population-based samples of elderly people. In this Review, we focus on these recent developments and their effects on two main questions: how CMBs are detected, and how CMBs should be interpreted. The number of CMBs detected depends on MRI characteristics, such as pulse sequence, sequence parameters, spatial resolution, magnetic field strength, and image post-processing, emphasising the importance of taking into account MRI technique in the interpretation of study results. Recent investigations with sensitive MRI techniques have indicated a high prevalence of CMBs in community-dwelling elderly people. We propose a procedural guide for identification of CMBs and suggest possible future approaches for elucidating the role of these common lesions as markers for, and contributors to, small-vessel brain disease.
Conflict of interest statement
The authors have no conflicts of interest relevant to the contents of the submitted manuscript.
Figures

References
-
- Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52(5):991–4. - PubMed
-
- Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70(14):1208–14. - PubMed
-
- Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66(2):165–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

