Purification and characterization of a small cationic protein from the tobacco hornworm Manduca sexta
- PMID: 19162182
- PMCID: PMC2659724
- DOI: 10.1016/j.ibmb.2008.12.006
Purification and characterization of a small cationic protein from the tobacco hornworm Manduca sexta
Abstract
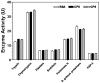
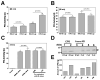
The prophenoloxidase (proPO) activation system is an important defense mechanism in arthropods, and activation of proPO to active phenoloxidase (PO) involves a serine proteinase cascade. Here, we report the purification and characterization of a small cationic protein CP8 from the tobacco hornworm, Manduca sexta, which can stimulate proPO activation. BLAST search showed that Manduca CP8 is similar to a fungal proteinase inhibitor-1 (AmFPI-1), an inducible serine proteinase inhibitor-1 (ISPI-1), and other small cationic proteins with unknown functions. However, we showed that Manduca CP8 did not inhibit proteinase activity, but stimulated proPO activation in plasma. When small amount (0.1 microg) of purified native CP8 or BSA was added to cell-free plasma samples and incubated for 20 min, low PO activity was observed in both groups. But significantly higher PO activity was observed in the CP8-group than in the BSA-group when more proteins (0.5 microg) were added and incubated for 20 min. However, when the plasma samples were incubated with proteins for 30 min, high PO activity was observed in both the CP8 and BSA groups regardless of the amount of proteins added. Moreover, when PO in the plasma was pre-activated with Micrococcus luteus, addition of CP8 did not have an effect on PO activity, and CP8/bacteria mixture did not stimulate PO activity to a higher level than did BSA/bacteria. These results suggest that CP8 helps activate proPO more rapidly at the initial stage. CP8 mRNA was specifically expressed in fat body and its mRNA level decreased when larvae were injected with saline or bacteria. However, CP8 protein concentration in hemolymph did not change significantly in larvae injected with saline or microorganisms.
Figures



Similar articles
-
Recognition of microbial molecular patterns and stimulation of prophenoloxidase activation by a β-1,3-glucanase-related protein in Manduca sexta larval plasma.Insect Biochem Mol Biol. 2011 May;41(5):322-31. doi: 10.1016/j.ibmb.2011.01.010. Epub 2011 Feb 4. Insect Biochem Mol Biol. 2011. PMID: 21296155 Free PMC article.
-
Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta.Insect Biochem Mol Biol. 2003 Feb;33(2):197-208. doi: 10.1016/s0965-1748(02)00191-1. Insect Biochem Mol Biol. 2003. PMID: 12535678
-
Manduca sexta serpin-7, a putative regulator of hemolymph prophenoloxidase activation.Insect Biochem Mol Biol. 2013 Jul;43(7):555-61. doi: 10.1016/j.ibmb.2013.03.015. Epub 2013 Apr 6. Insect Biochem Mol Biol. 2013. PMID: 23567587 Free PMC article.
-
Reconstitution of a branch of the Manduca sexta prophenoloxidase activation cascade in vitro: snake-like hemolymph proteinase 21 (HP21) cleaved by HP14 activates prophenoloxidase-activating proteinase-2 precursor.Insect Biochem Mol Biol. 2007 Oct;37(10):1015-25. doi: 10.1016/j.ibmb.2007.05.013. Epub 2007 May 29. Insect Biochem Mol Biol. 2007. PMID: 17785189 Free PMC article.
-
Innate immune responses of a lepidopteran insect, Manduca sexta.Immunol Rev. 2004 Apr;198:97-105. doi: 10.1111/j.0105-2896.2004.0121.x. Immunol Rev. 2004. PMID: 15199957 Review.
Cited by
-
The extended loop of the C-terminal carbohydrate-recognition domain of Manduca sexta immulectin-2 is important for ligand binding and functions.Amino Acids. 2012 Jun;42(6):2383-91. doi: 10.1007/s00726-011-0980-5. Epub 2011 Jul 30. Amino Acids. 2012. PMID: 21805136 Free PMC article.
-
Serpin7 controls egg diapause of migratory locust (Locusta migratoria) by regulating polyphenol oxidase.FEBS Open Bio. 2020 May;10(5):707-717. doi: 10.1002/2211-5463.12825. Epub 2020 Mar 24. FEBS Open Bio. 2020. PMID: 32107869 Free PMC article.
-
Characterization and expression profiling of serine protease inhibitors in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae).BMC Genomics. 2017 Feb 14;18(1):162. doi: 10.1186/s12864-017-3583-z. BMC Genomics. 2017. PMID: 28196471 Free PMC article.
-
Molecular Cloning and Functional Studies of Two Kazal-Type Serine Protease Inhibitors Specifically Expressed by Nasonia vitripennis Venom Apparatus.Toxins (Basel). 2015 Aug 4;7(8):2888-905. doi: 10.3390/toxins7082888. Toxins (Basel). 2015. PMID: 26248077 Free PMC article.
-
Isolation, characterization, kinetics, and enzymatic and nonenzymatic microbicidal activities of a novel c-type lysozyme from plasma of Schistocerca gregaria (Orthoptera: Acrididae).J Insect Sci. 2015 May 13;15(1):57. doi: 10.1093/jisesa/iev038. Print 2015. J Insect Sci. 2015. PMID: 25972507 Free PMC article.
References
-
- Ao JQ, Ling E, Yu XQ. A Toll receptor from Manduca sexta is in response to Escherichia coli infection. Mol Immunol. 2008;45:543–552. - PubMed
-
- Asgari S, Zhang G, Zareie R, Schmidt O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem Mol Biol. 2003;33:1017–1024. - PubMed
-
- Bradford M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

