A novel bicistronic vector for overexpressing Mycobacterium tuberculosis proteins in Escherichia coli
- PMID: 19162193
- PMCID: PMC2747733
- DOI: 10.1016/j.pep.2008.12.013
A novel bicistronic vector for overexpressing Mycobacterium tuberculosis proteins in Escherichia coli
Abstract
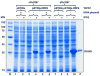
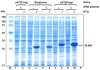
A putative DNA glycosylase encoded by the Rv3297 gene (MtuNei2) has been identified in Mycobacterium tuberculosis. Our efforts to express this gene in Escherichia coli either by supplementing tRNAs for rare codons or optimizing the gene with preferred codons for E. coli resulted in little or no expression. On the other hand, high-level expression was observed using a bicistronic expression vector in which the target gene was translationally coupled to an upstream leader sequence. Further comparison of the predicted mRNA secondary structures supported the hypothesis that mRNA secondary structure(s) surrounding the translation initiation region (TIR), rather than codon usage, played the dominant role in influencing translation efficiency, although manipulation of codon usage or tRNA supplementation did further enhance expression in the bicistronic vector. Addition of a cleavable N-terminal tag also facilitated gene expression in E. coli, possibly through a similar mechanism. However, since cleavage of N-terminal tags is determined by the amino acid at the P(1)' position downstream of the protease recognition sequence and results in the addition of an extra amino acid in front of the N-terminus of the protein, this strategy is not particularly amenable to Fpg/Nei family DNA glycosylases which carry the catalytic proline residue at the P(1)' position and require a free N-terminus. On the other hand, the bicistronic vector constructed here is potentially valuable particularly when expressing proteins from G/C rich organisms and when the proteins carry proline residues at the N-terminus in their native form. Thus the bicistronic expression system can be used to improve translation efficiency of mRNAs and achieve high-level expression of mycobacterial genes in E. coli.
Figures




Similar articles
-
A bicistronic vector with destabilized mRNA secondary structure yields scalable higher titer expression of human neurturin in E. coli.Biotechnol Bioeng. 2017 Aug;114(8):1753-1761. doi: 10.1002/bit.26299. Epub 2017 May 18. Biotechnol Bioeng. 2017. PMID: 28369693
-
The oxidative DNA glycosylases of Mycobacterium tuberculosis exhibit different substrate preferences from their Escherichia coli counterparts.DNA Repair (Amst). 2010 Feb 4;9(2):177-90. doi: 10.1016/j.dnarep.2009.11.008. Epub 2009 Dec 23. DNA Repair (Amst). 2010. PMID: 20031487 Free PMC article.
-
Construction of a modified vector for efficient purification of recombinant Mycobacterium tuberculosis proteins expressed in Escherichia coli.Protein Expr Purif. 2003 Jun;29(2):167-75. doi: 10.1016/s1046-5928(03)00052-4. Protein Expr Purif. 2003. PMID: 12767806
-
Enhanced production of recombinant Mycobacterium tuberculosis antigens in Escherichia coli by replacement of low-usage codons.Infect Immun. 2000 Jan;68(1):233-8. doi: 10.1128/IAI.68.1.233-238.2000. Infect Immun. 2000. PMID: 10603393 Free PMC article.
-
[Non-fused expression of HAb18GEF by reducing stability of translational initiation region in mRNA].Sheng Wu Gong Cheng Xue Bao. 2004 Mar;20(2):175-80. Sheng Wu Gong Cheng Xue Bao. 2004. PMID: 15969104 Chinese.
Cited by
-
Repair of oxidatively induced DNA damage by DNA glycosylases: Mechanisms of action, substrate specificities and excision kinetics.Mutat Res Rev Mutat Res. 2017 Jan-Mar;771:99-127. doi: 10.1016/j.mrrev.2017.02.001. Epub 2017 Feb 16. Mutat Res Rev Mutat Res. 2017. PMID: 28342455 Free PMC article. Review.
-
Expression and purification of active mouse and human NEIL3 proteins.Protein Expr Purif. 2012 Jul;84(1):130-9. doi: 10.1016/j.pep.2012.04.022. Epub 2012 May 5. Protein Expr Purif. 2012. PMID: 22569481 Free PMC article.
-
Novel methods to optimize gene and statistic test for evaluation - an application for Escherichia coli.BMC Bioinformatics. 2017 Feb 10;18(1):100. doi: 10.1186/s12859-017-1517-z. BMC Bioinformatics. 2017. PMID: 28187713 Free PMC article.
-
Bicistronic design as recombinant expression enhancer: characteristics, applications, and structural optimization.Appl Microbiol Biotechnol. 2021 Oct;105(20):7709-7720. doi: 10.1007/s00253-021-11611-5. Epub 2021 Oct 1. Appl Microbiol Biotechnol. 2021. PMID: 34596722 Review.
-
Codon preference optimization increases heterologous PEDF expression.PLoS One. 2010 Nov 30;5(11):e15056. doi: 10.1371/journal.pone.0015056. PLoS One. 2010. PMID: 21152082 Free PMC article.
References
-
- Andersson GE, Sharp PM. Codon usage in the Mycobacterium tuberculosis complex. Microbiology (Reading, England) 1996;142(4):915–925. - PubMed
-
- Hannig G, Makrides SC. Strategies for optimizing heterologous protein expression in Escherichia coli. Trends in Biotechnology. 1998;16:54–60. - PubMed
-
- Kane JF. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Current Opinion in Biotechnology. 1995;6:494–500. - PubMed
-
- Kurland C, Gallant J. Errors of heterologous protein expression. Current Opinion in Biotechnology. 1996;7:489–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

