Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash
- PMID: 19162309
- PMCID: PMC2741166
- DOI: 10.1016/j.ygyno.2008.12.003
Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash
Abstract
Objectives: Determine if cetuximab dose escalation to induce grade 2 rash correlates with anti-tumor activity and if sera-based markers could predict likelihood of response.
Methods: Patients with persistent/recurrent ovarian or primary peritoneal carcinoma received an initial dose of cetuximab 400 mg/m(2), then 250 mg/m(2) weekly for two 3-week cycles. Patients who had stable disease (SD) and <grade 2 rash were dose escalated in 75 mg/m(2) increments every 3 weeks until grade 2 rash or to a maximum weekly dose of 400 mg/m(2). Pre- and post-treatment serum samples were evaluated for potential predictive markers of response.
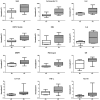
Results: One of 25 patients achieved partial remission (PR) and 9 patients had SD. The median progression free survival was 2.1 months; the 1-year survival rate was 54.8%. Rash (96%) was the most common drug-related adverse event. At first response assessment, 4 patients remained at 250 mg/m(2); 8 patients were dose-escalated to 325 mg/m(2); of these, 4 ultimately were increased to 400 mg/m(2). Patients with progressive disease (PD) were removed from the study. Ninety-two serologic markers were analyzed from 20 patients to identify markers associated with clinical activity and/or predictive of outcome. Pretreatment levels of twelve markers were significantly elevated in patients exhibiting PD versus SD or PR; however, changes in marker levels during the course of treatment were not significant indicators of response.
Conclusions: Single-agent cetuximab showed minimal activity in patients with recurrent ovarian cancer. Patients with elevated levels of 12 serologic markers at baseline were more likely to have earlier disease progression.
Figures

References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Ozols RFRS, Thomas GM, Robboy SJ. Epithelial ovarian cancer. In: Hoskins WJPC, Young RC, Barakat RR, Markman M, Randall ME, editors. Principles and practice of gynecologic oncology. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 895–987.
-
- Bookman MA, for the Gynecologic Cancer InterGroup through the Gynecologic Oncology G GOG0182-ICON5: 5-arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG-lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced-stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. ASCO Meet Abstr. 2006;24:5002.
-
- Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–611. - PubMed
-
- Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785:232–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

