Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules
- PMID: 19162317
- PMCID: PMC2921510
- DOI: 10.1016/j.biomaterials.2009.01.007
Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules
Abstract
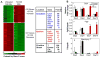
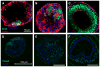
Cell specification and tissue formation during embryonic development are precisely controlled by the local concentration and temporal presentation of morphogenic factors. Similarly, pluripotent embryonic stem cells can be induced to differentiate in vitro into specific phenotypes in response to morphogen treatment. Embryonic stem cells (ESCs) are commonly differentiated as 3D spheroids referred to as embryoid bodies (EBs); however, differentiation of cells within EBs is typically heterogeneous and disordered. In this study, we demonstrate that in contrast to soluble morphogen treatment, delivery of morphogenic factors directly within EB microenvironments in a spatiotemporally controlled manner using polymer microspheres yields homogeneous, synchronous and organized ESC differentiation. Degradable PLGA microspheres releasing retinoic acid were incorporated directly within EBs and induced the formation of cystic spheroids uniquely resembling the phenotype and structure of early streak mouse embryos (E6.75), with an exterior of FOXA2+ visceral endoderm enveloping an epiblast-like layer of OCT4+ cells. These results demonstrate that controlled morphogen presentation to stem cells using degradable microspheres more efficiently directs cell differentiation and tissue formation than simple soluble delivery methods and presents a unique route to study the spatiotemporal effects of morphogenic factors on embryonic developmental processes in vitro.
Figures




Similar articles
-
Microsphere size effects on embryoid body incorporation and embryonic stem cell differentiation.J Biomed Mater Res A. 2010 Aug;94(2):466-75. doi: 10.1002/jbm.a.32710. J Biomed Mater Res A. 2010. PMID: 20213812
-
Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation.Biotechnol Prog. 2009 Jan-Feb;25(1):43-51. doi: 10.1002/btpr.139. Biotechnol Prog. 2009. PMID: 19198003 Free PMC article. Review.
-
Assessment of differentiation aspects by the morphological classification of embryoid bodies derived from human embryonic stem cells.Stem Cells Dev. 2011 Nov;20(11):1925-35. doi: 10.1089/scd.2010.0476. Epub 2011 Apr 19. Stem Cells Dev. 2011. PMID: 21388292
-
Cluster characterization of mouse embryonic stem cell-derived pluripotent embryoid bodies in four distinct developmental stages.Biologicals. 2009 Aug;37(4):235-44. doi: 10.1016/j.biologicals.2009.03.001. Epub 2009 Mar 31. Biologicals. 2009. PMID: 19339198
-
Microfluidic-based patterning of embryonic stem cells for in vitro development studies.Lab Chip. 2013 Dec 7;13(23):4617-24. doi: 10.1039/c3lc50663k. Lab Chip. 2013. PMID: 24113509 Free PMC article.
Cited by
-
On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology.Biomaterials. 2015 Jun;52:26-43. doi: 10.1016/j.biomaterials.2015.01.078. Epub 2015 Feb 21. Biomaterials. 2015. PMID: 25818411 Free PMC article. Review.
-
Microfluidic sorting of microtissues.Biomicrofluidics. 2012 Mar;6(1):14116-1411611. doi: 10.1063/1.3692765. Epub 2012 Mar 7. Biomicrofluidics. 2012. PMID: 22505992 Free PMC article.
-
Accelerated and Improved Differentiation of Retinal Organoids from Pluripotent Stem Cells in Rotating-Wall Vessel Bioreactors.Stem Cell Reports. 2018 Jan 9;10(1):300-313. doi: 10.1016/j.stemcr.2017.11.001. Epub 2017 Dec 7. Stem Cell Reports. 2018. PMID: 29233554 Free PMC article.
-
Droplet microfluidic devices for organized stem cell differentiation into germ cells: capabilities and challenges.Biophys Rev. 2021 Nov 17;13(6):1245-1271. doi: 10.1007/s12551-021-00907-5. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35059040 Free PMC article. Review.
-
Optogenetic control of Wnt signaling models cell-intrinsic embryogenic patterning using 2D human pluripotent stem cell culture.Development. 2023 Jul 15;150(14):dev201386. doi: 10.1242/dev.201386. Epub 2023 Jul 26. Development. 2023. PMID: 37401411 Free PMC article.
References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. - PubMed
-
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. - PubMed
-
- Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, et al. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

