Dissecting components of reward: 'liking', 'wanting', and learning
- PMID: 19162544
- PMCID: PMC2756052
- DOI: 10.1016/j.coph.2008.12.014
Dissecting components of reward: 'liking', 'wanting', and learning
Abstract
In recent years significant progress has been made delineating the psychological components of reward and their underlying neural mechanisms. Here we briefly highlight findings on three dissociable psychological components of reward: 'liking' (hedonic impact), 'wanting' (incentive salience), and learning (predictive associations and cognitions). A better understanding of the components of reward, and their neurobiological substrates, may help in devising improved treatments for disorders of mood and motivation, ranging from depression to eating disorders, drug addiction, and related compulsive pursuits of rewards.
Figures




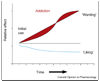
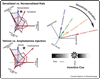
References
-
- Schooler JW, Mauss IB. To be happy and to know it: the experience and meta-awareness of pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; in press.
-
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull. 2005;31:121–135. - PubMed
-
- Fischman MW, Foltin RW. Self-administration of cocaine by humans: a laboratory perspective. In: Bock GR, Whelan J, editors. Cocaine: Scientific and Social Dimensions. CIBA Foundation Symposium; Wiley; 1992. pp. 165–180. - PubMed
-
-
Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702.Vividly and concisely describes the role of orbitofrontal cortex role in pleasure in humans.
-
-
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

