Comparative genomics of aldehyde dehydrogenase 5a1 (succinate semialdehyde dehydrogenase) and accumulation of gamma-hydroxybutyrate associated with its deficiency
- PMID: 19164088
- PMCID: PMC2657722
- DOI: 10.1186/1479-7364-3-2-106
Comparative genomics of aldehyde dehydrogenase 5a1 (succinate semialdehyde dehydrogenase) and accumulation of gamma-hydroxybutyrate associated with its deficiency
Abstract
Succinic semialdehyde dehydrogenase (SSADH; aldehyde dehydrogenase 5A1 [ALDH5A1]; locus 6p22) occupies a central position in central nervous system (CNS) neurotransmitter metabolism as one of two enzymes necessary for gamma-aminobutyric acid (GABA) recycling from the synaptic cleft. Its importance is highlighted by the neurometabolic disease associated with its inherited deficiency in humans, as well as the severe epileptic phenotype observed in Aldh5a1(-/-) knockout mice. Expanding evidence now suggests, however, that even subtle decreases in human SSADH activity, associated with rare and common single nucleotide polymorphisms, may produce subclinical pathological effects. SSADH, in conjunction with aldo-keto reductase 7A2 (AKR7A2), represent two neural enzymes responsible for further catabolism of succinic semialdehyde, producing either succinate (SSADH) or gamma-hydroxybutyrate (GHB; AKR7A2). A GABA analogue, GHB is a short-chain fatty alcohol with unusual properties in the CNS and a long pharmacological history. Moreover, SSADH occupies a further role in the CNS as the enzyme responsible for further metabolism of the lipid peroxidation aldehyde 4-hydroxy-2-nonenal (4-HNE), an intermediate known to induce oxidant stress. Accordingly, subtle decreases in SSADH activity may have the capacity to lead to regional accumulation of neurotoxic intermediates (GHB, 4-HNE). Polymorphisms in SSADH gene structure may also associate with quantitative traits, including intelligence quotient and life expectancy. Further population-based studies of human SSADH activity promise to reveal additional properties of its function and additional roles in CNS tissue.
Figures
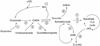


Similar articles
-
Mutants of GABA transaminase (POP2) suppress the severe phenotype of succinic semialdehyde dehydrogenase (ssadh) mutants in Arabidopsis.PLoS One. 2008;3(10):e3383. doi: 10.1371/journal.pone.0003383. Epub 2008 Oct 10. PLoS One. 2008. PMID: 18846220 Free PMC article.
-
Succinic semialdehyde dehydrogenase deficiency (SSADHD): Pathophysiological complexity and multifactorial trait associations in a rare monogenic disorder of GABA metabolism.Neurochem Int. 2016 Oct;99:72-84. doi: 10.1016/j.neuint.2016.06.009. Epub 2016 Jun 14. Neurochem Int. 2016. PMID: 27311541 Free PMC article. Review.
-
Temporal metabolomics in dried bloodspots suggests multipathway disruptions in aldh5a1-/- mice, a model of succinic semialdehyde dehydrogenase deficiency.Mol Genet Metab. 2019 Dec;128(4):397-408. doi: 10.1016/j.ymgme.2019.10.003. Epub 2019 Oct 31. Mol Genet Metab. 2019. PMID: 31699650 Review.
-
Enzymatic and metabolic evidence for a region specific mitochondrial dysfunction in brains of murine succinic semialdehyde dehydrogenase deficiency (Aldh5a1-/- mice).Neurochem Int. 2007 Mar;50(4):653-9. doi: 10.1016/j.neuint.2006.12.009. Epub 2007 Jan 13. Neurochem Int. 2007. PMID: 17303287
-
Therapeutic concepts in succinate semialdehyde dehydrogenase (SSADH; ALDH5a1) deficiency (gamma-hydroxybutyric aciduria). Hypotheses evolved from 25 years of patient evaluation, studies in Aldh5a1-/- mice and characterization of gamma-hydroxybutyric acid pharmacology.J Inherit Metab Dis. 2007 Jun;30(3):279-94. doi: 10.1007/s10545-007-0574-2. Epub 2007 Apr 24. J Inherit Metab Dis. 2007. PMID: 17457693
Cited by
-
Single-Cell Patch-Clamp/Proteomics of Human Alzheimer's Disease iPSC-Derived Excitatory Neurons Versus Isogenic Wild-Type Controls Suggests Novel Causation and Therapeutic Targets.Adv Sci (Weinh). 2024 Aug;11(29):e2400545. doi: 10.1002/advs.202400545. Epub 2024 May 21. Adv Sci (Weinh). 2024. PMID: 38773714 Free PMC article.
-
Pharmacokinetics and pharmacodynamics of γ-hydroxybutyrate in healthy subjects.Br J Clin Pharmacol. 2016 May;81(5):980-8. doi: 10.1111/bcp.12863. Epub 2016 Feb 25. Br J Clin Pharmacol. 2016. PMID: 26659543 Free PMC article. Clinical Trial.
-
Novel ALDH5A1 variants and genotype: Phenotype correlation in SSADH deficiency.Neurology. 2020 Nov 10;95(19):e2675-e2682. doi: 10.1212/WNL.0000000000010730. Epub 2020 Sep 4. Neurology. 2020. PMID: 32887777 Free PMC article.
-
Proteomic analysis of cardiac ventricles: baso-apical differences.Mol Cell Biochem. 2018 Aug;445(1-2):211-219. doi: 10.1007/s11010-017-3266-8. Epub 2018 Jan 4. Mol Cell Biochem. 2018. PMID: 29302836
-
The Drug of Abuse Gamma-Hydroxybutyric Acid Exhibits Tissue-Specific Nonlinear Distribution.AAPS J. 2017 Dec 26;20(1):21. doi: 10.1208/s12248-017-0180-7. AAPS J. 2017. PMID: 29280004 Free PMC article.
References
-
- Hedou G, Chasserot-Golaz S, Kemmel V. et al.Immunohistochemical studies of the localization of neurons containing the enzyme that synthesizes dopamine, GABA, or gamma-hydroxybutyrate in the rat substantia nigra and striatum. J Comp Neurol. 2000;426:549–560. doi: 10.1002/1096-9861(20001030)426:4<549::AID-CNE4>3.0.CO;2-Y. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

