A novel role for embigin to promote sprouting of motor nerve terminals at the neuromuscular junction
- PMID: 19164284
- PMCID: PMC2659250
- DOI: 10.1074/jbc.M809491200
A novel role for embigin to promote sprouting of motor nerve terminals at the neuromuscular junction
Abstract
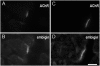
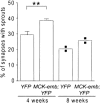
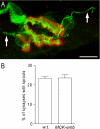
Adult skeletal muscle accepts ectopic innervation by foreign motor axons only after section of its own nerve, suggesting that the formation of new neuromuscular junctions is promoted by muscle denervation. With the aim to identify new proteins involved in neuromuscular junction formation we performed an mRNA differential display on innervated versus denervated adult rat muscles. We identified transcripts encoding embigin, a transmembrane protein of the immunoglobulin superfamily (IgSF) class of cell adhesion molecules to be strongly regulated by the state of innervation. In innervated muscle it is preferentially localized to neuromuscular junctions. Forced overexpression in innervated muscle of a full-length embigin transgene, but not of an embigin fragment lacking the intracellular domain, promotes nerve terminal sprouting and the formation of additional acetylcholine receptor clusters at synaptic sites without affecting terminal Schwann cell number or morphology, and it delays the retraction of terminal sprouts following re-innervation of denervated endplates. Conversely, knockdown of embigin by RNA interference in wild-type muscle accelerates terminal sprout retraction, both by itself and synergistically with deletion of neural cell adhesion molecule. These findings indicate that embigin enhances neural cell adhesion molecule-dependent neuromuscular adhesion and thereby modulates neuromuscular junction formation and plasticity.
Figures





References
-
- Bezakova, G., and Ruegg, M. A. (2003) Nat. Rev. Mol. Cell. Biol. 4 295-308 - PubMed
-
- Lømo, T. (2003) J. Neurocytol. 32 835-848 - PubMed
-
- Bowen, D. C., Park, J. S., Bodine, S., Stark, J. L., Valenzuela, D. M., Stitt, T. N., Yancopoulos, G. D., Lindsay, R. M., Glass, D. J., and DiStefano, P. S. (1998) Dev. Biol. 199 309-319 - PubMed
-
- Meier, T., Marangi, P. A., Moll, J., Hauser, D. M., Brenner, H. R., and Ruegg, M. A. (1998) Eur. J. Neurosci. 10 3141-3152 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

