Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury
- PMID: 19164288
- PMCID: PMC2658078
- DOI: 10.1074/jbc.M808824200
Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury
Abstract
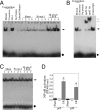
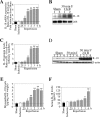
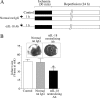
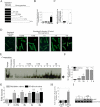
Ischemia/reperfusion (I/R) injury is characterized by the induction of oxidative stress and proinflammatory cytokine expression. Recently demonstrating that oxidative stress and TNF-alpha each stimulate interleukin (IL)-18 expression in cardiomyocytes, we hypothesized that I/R also induces IL-18 expression and thus exacerbates inflammation and tissue damage. Neutralization of IL-18 signaling should therefore diminish tissue injury following I/R. I/R studies were performed using a chronically instrumented closed chest mouse model. Male C57BL/6 mice underwent 30 min of ischemia by LAD coronary artery ligation followed by various periods of reperfusion. Sham-operated or ischemia-only mice served as controls. A subset of animals was treated with IL-18-neutralizing antibodies 1 h prior to LAD ligation. Ischemic LV tissue was used for analysis. Our results demonstrate that, compared with sham operation and ischemia alone, I/R significantly increased (i) oxidative stress (increased MDA/4-HNE levels), (ii) neutrophil infiltration (increased MPO activity), (iii) NF-kappaB DNA binding activity (p50, p65), and (iv) increased expression of IL-18Rbeta, but not IL-18Ralpha or IL-18BP transcripts. Administration of IL-18-neutralizing antibodies significantly reduced I/R injury measured by reduced infarct size (versus control IgG). In isolated adult mouse cardiomyocytes, simulated ischemia/reperfusion enhanced oxidative stress and biologically active IL-18 expression via IKK-dependent NF-kappaB activation. These results indicate that IL-18 plays a critical role in I/R injury and thus represents a promising therapeutic target.
Figures

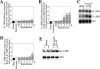
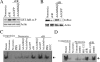

References
-
- Okamura, H., Tsutsi, H., Komatsu, T., Yutsudo, M., Hakura, A., Tanimoto, T., Torigoe, K., Okura, T., Nukada, Y., Hattori, K., Akita, K., Namba, M., Tanabe, F., Konishi, K., Fukuda, S., and Kurimoto, M. (1995) Nature 378 88-91 - PubMed
-
- Gracie, J. A., Robertson, S. E., and McInnes, I. B. (2003) J. Leukocyte Biol. 73 213-224 - PubMed
-
- Dinarello, C. A., Novick, D., Puren, A. J., Fantuzzi, G., Shapiro, L., Muhl, H., Yoon, D. Y., Reznikov, L. L., Kim, S. H., and Rubinstein, M. (1998) J. Leukocyte Biol. 63 658-664 - PubMed
-
- Wang, M., Markel, T. A., and Meldrum, D. R. (2008) Shock 30 3-10 - PubMed
-
- Arend, W. P., Palmer, G., and Gabay, C. (2008) Immunol. Rev. 223 20-38 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

