N-Glycan Moieties in Neonatal Fc Receptor Determine Steady-state Membrane Distribution and Directional Transport of IgG
- PMID: 19164298
- PMCID: PMC2659187
- DOI: 10.1074/jbc.M805877200
N-Glycan Moieties in Neonatal Fc Receptor Determine Steady-state Membrane Distribution and Directional Transport of IgG
Abstract
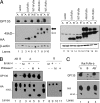
The neonatal Fc receptor (FcRn) is a major histocompatibility complex class I-related molecule known to protect IgG and albumin from catabolism and transport IgG across polarized epithelial cells in a bidirectional manner. Previous studies have shown species-specific differences in ligand binding, IgG transport direction, and steady-state membrane distribution when expressed in polarized epithelial cells. We hypothesized that these differences may be due to the additional N-glycans expressed on the rat FcRn, because N-glycans have been proposed to function as apical targeting signals, and that two of the N-glycan moieties have been shown to contribute to the IgG binding of rat FcRn. A panel of mutant human FcRn variants was generated to resemble the N-glycan expression of rat FcRn in various combinations and subsequently transfected into Madin-Darby canine kidney II cells together with human beta2-microglobulin. Mutant human FcRn clones that contained additional N-glycan side-chain modifications, including that which was fully rodentized, still exhibited specificity for human IgG and failed to bind to mouse IgG. At steady state, the mutant human FcRn with additional N-glycans redistributed to the apical cell surface similar to that of rat FcRn. Furthermore, the rodentized human FcRn exhibited a reversal of IgG transport with predominant transcytosis from an apical-to-basolateral direction, which resembled that of the rat FcRn isoform. These studies show that the N-glycans in FcRn contribute significantly to the steady-state membrane distribution and direction of IgG transport in polarized epithelia.
Figures



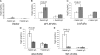
References
-
- Brambell, F. W. (1969) Proc. Nutr. Soc. 28 35–41 - PubMed
-
- Simister, N. E., and Mostov, K. E. (1989) Cold Spring Harbor Symp. Quant. Biol. 54 571–580 - PubMed
-
- Yoshida, M., Masuda, A., Kuo, T. T., Kobayashi, K., Claypool, S. M., Takagawa, T., Kutsumi, H., Azuma, T., Lencer, W. I., and Blumberg, R. S. (2006) Springer Semin. Immunopathol. 28 397–403 - PubMed
-
- Burmeister, W. P., Huber, A. H., and Bjorkman, P. J. (1994) Nature 372 379–383 - PubMed
-
- Rodewald, R., Lewis, D. M., and Kraehenbuhl, J. P. (1983) CIBA Found. Symp. 95 287–299 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

