Insulin regulates TRB3 and other stress-responsive gene expression through induction of C/EBPbeta
- PMID: 19164449
- PMCID: PMC5419275
- DOI: 10.1210/me.2008-0284
Insulin regulates TRB3 and other stress-responsive gene expression through induction of C/EBPbeta
Abstract
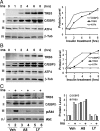
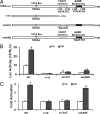
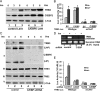
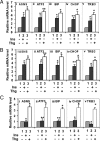
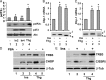
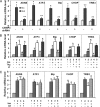
Pseudokinase TRB3 is an inducible gene whose expression is regulated by stress response and insulin and associated with insulin resistance and metabolic syndrome. In this report, we have investigated the mechanism under which insulin regulates TRB3 gene expression and demonstrated that insulin induces TRB3 expression via C/EBPbeta. We found that in Fao hepatoma and 3T3-L1 adipocytes, C/EBPbeta expression induced by insulin preceded that of TRB3 and that mutation of the C/EBPbeta binding site in TRB3 promoter abolished the responsiveness of the TRB3 gene to insulin. We further showed that ectopic expression of C/EBPbeta augmented, whereas knockdown of C/EBPbeta reduced, TRB3 expression induced by insulin. In addition, we presented data to show that insulin, through a similar mechanism under which insulin induces TRB3 expression, promotes the expression of genes such as ANAS, ATF3, BIP, and CHOP, which are typical stress-responsive genes. We also examined the impact of C/EBPbeta expression on Akt activation and found that inaction of C/EBPbeta not only augmented Akt activation but also obliterated the suppression of Akt activation due to prolonged insulin stimulation. We suggest, through induction of C/EBPbeta in hepatic cells and adipocytes, that insulin induces the expression of stress-responsive genes, which may represent a novel insulin action.
Figures

Similar articles
-
TRB3 blocks adipocyte differentiation through the inhibition of C/EBPbeta transcriptional activity.Mol Cell Biol. 2007 Oct;27(19):6818-31. doi: 10.1128/MCB.00375-07. Epub 2007 Jul 23. Mol Cell Biol. 2007. PMID: 17646392 Free PMC article.
-
PI3K activates negative and positive signals to regulate TRB3 expression in hepatic cells.Exp Cell Res. 2008 Apr 15;314(7):1566-74. doi: 10.1016/j.yexcr.2008.01.026. Epub 2008 Feb 11. Exp Cell Res. 2008. PMID: 18316073
-
Glucose, dexamethasone, and the unfolded protein response regulate TRB3 mRNA expression in 3T3-L1 adipocytes and L6 myotubes.Am J Physiol Endocrinol Metab. 2006 Dec;291(6):E1274-80. doi: 10.1152/ajpendo.00117.2006. Epub 2006 Jul 11. Am J Physiol Endocrinol Metab. 2006. PMID: 16835403
-
Fibrates upregulate TRB3 in lymphocytes independent of PPAR alpha by augmenting CCAAT/enhancer-binding protein beta (C/EBP beta) expression.Mol Immunol. 2007 Feb;44(6):1218-29. doi: 10.1016/j.molimm.2006.06.006. Epub 2006 Sep 1. Mol Immunol. 2007. PMID: 16949670
-
Multiple mechanisms of growth hormone-regulated gene transcription.Mol Genet Metab. 2007 Feb;90(2):126-33. doi: 10.1016/j.ymgme.2006.10.006. Epub 2006 Nov 28. Mol Genet Metab. 2007. PMID: 17129742 Free PMC article. Review.
Cited by
-
Cellular stress induces TRB3/USP9x-dependent Notch activation in cancer.Oncogene. 2017 Feb 23;36(8):1048-1057. doi: 10.1038/onc.2016.276. Epub 2016 Sep 5. Oncogene. 2017. PMID: 27593927
-
The contribution of endoplasmic reticulum stress to liver diseases.Hepatology. 2011 May;53(5):1752-63. doi: 10.1002/hep.24279. Hepatology. 2011. PMID: 21384408 Free PMC article.
-
Control of adipose tissue inflammation through TRB1.Diabetes. 2010 Aug;59(8):1991-2000. doi: 10.2337/db09-1537. Epub 2010 Jun 3. Diabetes. 2010. PMID: 20522600 Free PMC article.
-
Evaluation of sestrin 2 and tribbles homolog 3 levels in obese and nonobese women with polycystic ovary syndrome.Turk J Med Sci. 2023 Dec 4;53(6):1697-1703. doi: 10.55730/1300-0144.5738. eCollection 2023. Turk J Med Sci. 2023. PMID: 38813505 Free PMC article.
-
The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights.Mol Cell Endocrinol. 2011 Mar 15;335(1):2-13. doi: 10.1016/j.mce.2010.04.005. Epub 2010 Apr 14. Mol Cell Endocrinol. 2011. PMID: 20398732 Free PMC article. Review.
References
-
- Hegedus Z, Czibula A, Kiss-Toth E2007. Tribbles: a family of kinase-like proteins with potent signalling regulatory function. Cell Signal 19:238–250 - PubMed
-
- Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR2006. Emerging roles of pseudokinases. Trends Cell Biol 16:443–452 - PubMed
-
- Mata J, Curado S, Ephrussi A, Rorth P2000. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101:511–522 - PubMed
-
- Rorth P, Szabo K, Texido G2000. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol Cell 6:23–30 - PubMed
-
- Du K, Herzig S, Kulkarni RN, Montminy M2003. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300:1574–1577 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

